Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WU Xuwen, HUTCHINGS Pat, MURRAY Anna, XU Kuidong
- Laetmonice iocasica sp. nov., a new polychaete species (Annelida: Aphroditidae) from seamounts in the tropical Western Pacific, with remarks on L. producta Grube, 1877
- Journal of Oceanology and Limnology, 39(5): 1805-1816
- http://dx.doi.org/10.1007/s00343-021-0413-6
Article History
- Received Oct. 26, 2020
- accepted in principle Nov. 16, 2020
- accepted for publication Mar. 4, 2021
2 Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), Zhuhai 519082, China;
3 Australian Museum, Australian Museum Research Institute, Sydney NSW 2000, Australia;
4 Biological Sciences, Macquarie University, North Ryde 2109, Australia;
5 University of Chinese Academy of Sciences, Beijing 100049, China
Members in the family Aphroditidae are predominantly deep-water scale-worms, and few species occur intertidally (Hutchings and McRae, 1993). Within the family, the genus Laetmonice Kinberg, 1856 is characterized by the remarkable harpoon notochaetae which are distally tapering with several recurved fangs on lateral margins (Hutchings, 2000). Up to date, 27 species have been included in the genus, in which 24 species were originally described by early taxonomists more than a century ago (Read and Fauchald, 2021a). It is not surprising that as many as five generic names have been considered as synonymies of Laetmonice because the early descriptions are brief and not all important characters are mentioned (Hutchings and McRae, 1993; Barnich and Fiege, 2003).
Species of Laetmonice are highly diverse in the Indonesian Archipelago and Australian waters. Hutchings and McRae (1993) made an important revision of Laetmonice mainly based on the types of the Indo-Pacific and Australian species and provided a checklist of 18 species distributed in China, Philippines, Indonesia, New Guinea, and Australia including two new species. In contrast, only a few species of Laetmonice are reported in the Atlantic. Barnich and Fiege (2003) made a comprehensive work on the Mediterranean species of Laetmonice and only two valid species were found in the East Atlantic, Mediterranean Sea, and the northern part of the Red Sea. The most recent new record of Laetmonice is from Barnich et al. (2013), who described a new species associated with cold-water corals in the eastern Gulf of Mexico.
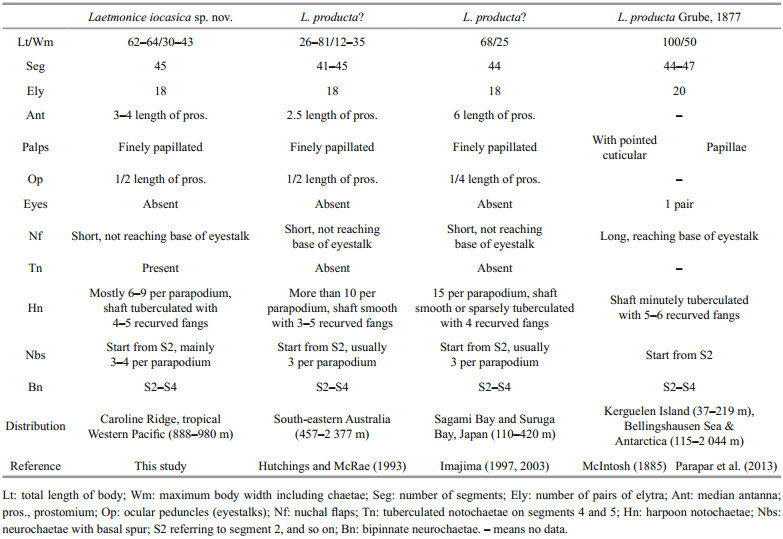
Based on the examination of polychaete specimens collected from the seamounts located on the Caroline Ridge in the tropical Western Pacific in 2019, we identified and described a new species of Aphroditidae: Laetmonice iocasica sp. nov. For species comparison, we evaluated the taxonomic status of the species Laetmonice producta Grube, 1877 and its varieties and subspecies, which were recorded in the Indian, Pacific, Atlantic, and Southern Oceans. The species Laetmonice producta was commonly found at high latitudes of the Southern Ocean (Grube, 1877; Parapar et al., 2013), and it is confusing that L. producta was reported from the Sagami Bay and Suruga Bay in Japan and the southeastern Australia (Hutchings and McRae, 1993; Imajima, 2003). In this study, we provided some remarks on the species L. producta, particularly its records in Japan and Australia.
2 MATERIAL AND METHOD 2.1 Specimen collection and morphological examinationSpecimens were collected from two adjacent seamounts (provisionally named as M5 and M6) located on the Caroline Ridge in the tropical Western Pacific (Fig. 1). A total of 3 specimens were obtained from three dives (140°11'E–140°14'E, 10°04'N–10°07'N, water depth of 888–980 m) by the ROV Faxian (Discovery in Chinese) on board the R/V Kexue (Science in Chinese) in June 2019. Photos were taken in situ by the imaging equipment of the ROV. The specimens were photographed on board using a Canon EOS camera, then preserved in 80% ethanol solution and later deposited in the Marine Biological Museum of Chinese Academy of Sciences (MBMCAS), Institute of Oceanology, Chinese Academy of Sciences (IOCAS). Details of prostomium, pygidium, segments, parapodia and chaetae were observed through a Zeiss Discovery. V20 stereomicroscope. Digital photographs were taken using an AxioCam 512 digital camera mounted on the microscope. Images of different focal planes were stacked by the software Helicon Focus 7. The character choice and terminology mainly followed Hutchings and McRae (1993).

|
| Fig.1 Sampling sites of Laetmonice iocasica sp. nov. Arrows pointing to two seamounts on the Caroline Ridge in the tropical Western Pacific. |
Genomic DNA was extracted from each of the three specimens using the TIANamp Marine Animal DNA Kit (Tiangen Bio. Co., Beijing, China). Approximately 650 bp of cytochrome c oxidase subunit I (COI), 400 bp of 16S, 1 700 bp of 18S, 1 000 bp of 28S, and 900 bp of ITS (ITS1-5.8S-ITS2) genes were amplified using the following primers: LoboF1 and LoboR1 for COI (Lobo et al., 2013); Ann16SF and Ann16SR for 16S (Sjölin et al., 2005); 18SA and 18SB for 18S (Medlin et al., 1998; Nygren and Sundberg, 2003), 28S D1_F and D3 for 28S (Brown et al., 1999; Vonnemann et al., 2005); ITS18SFPOLY and POLY_28R for ITS (Barfuss, 2012). The 25-μL reaction contained 12-μL 2X Es TaqMasterMix (CWBio Co., Ltd., Beijing, China), 1 μL of each primer (10 mmol/L), 2 μL of template DNA (50 ng/μL), and 9-μL dH2O. Thermal cycling protocols followed Wu et al. (2019). Amplified DNA was sequenced using the ABI 3730 DNA Analyzer sequencing facility of the Shanghai Sangon Biological Engineering and Technical Service Company, Shanghai, China.
2.3 Barcoding and phylogenetic analysesThree partial sequences of COI were obtained from each of the three specimens in this study and were deposited in GenBank. To examine species delimitation, all available COI sequences of Laetmonice species were downloaded from GenBank and used in the barcoding and phylogenetic analyses (Table 1). The outgroup comprised species of Aphrodita Linnaeus, 1758, Palmyra Savigny in Lamarck, 1818, Nereis Linnaeus, 1758, and Glycera Lamarck, 1818. The former two genera represented the other species of the family Aphroditidae available in GenBank. Sequences were aligned using ClustalW method provided in MEGA-X. Pairwise distances of COI were calculated using MEGA-X based on the Kimura 2-parameter and p-distance models (Kumar et al., 2018).
Maximum likelihood (ML) analysis was carried out using IQ-TREE (Nguyen et al., 2015) in PhyloSuite v1.2.2 (Zhang et al., 2020) under the model automatically selected by IQ-TREE ("Auto" option in IQ-TREE) for 5 000 ultrafast (Minh et al., 2013) bootstraps. Bayesian inference (BI) analysis was carried out using MrBayes 3.2.6 (Ronquist et al., 2012) under GTR+I+G model (2 parallel runs, four chains of 3 000 000 generations with sampling every 1 000 generations), in which the initial 25% of sampled data were discarded as burn-in trees.
3 RESULT 3.1 TaxonomyFamily Aphroditidae Malmgren, 1867
Genus Laetmonice Kinberg, 1856
Laetmonice iocasica sp. nov.

|
| Fig.2 Three specimens of Laetmonice iocasica sp. nov. holotype (a, d) and paratypes (b, e, MBM286067; c, f, MBM286068) a–c. three specimens in situ; d–f. three specimens in dorsal (left sides) and ventral view (right sides). Scale bars: 10 mm (d–f). |
|
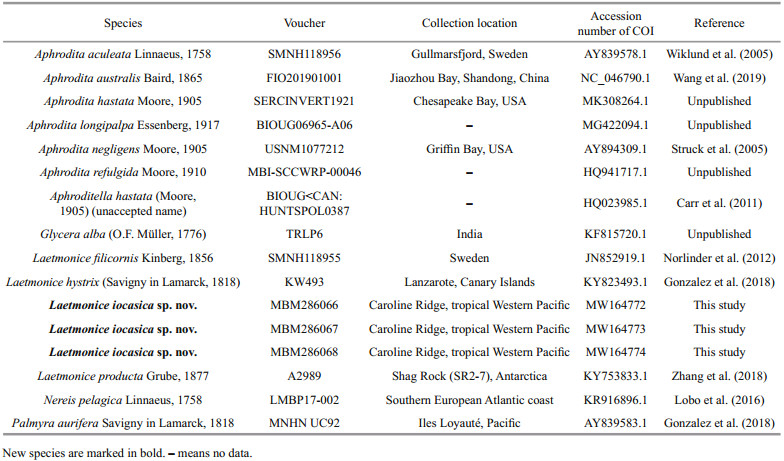
| Fig.3 Laetmonice iocasica sp. nov. holotype (c–f) and paratype (a, b, g–i, MBM286067) a, b. head and anterior segments in dorsal view (a) and ventral view (b); c. left side of median segments in dorsal view; d. left side of median segments in ventral view; e, i. posterior segments in dorsal (e) and ventral (i) view; f. posterior segments in ventral view; g. elytron from second segment; h. elytron from unknown position. Abbreviations: S2: referring to segment 2, and so on; an: acicular notochaetae; es: eyestalk; ft: facial tubercle; hn: harpoon notochaetae; nf: nuchal flap. Scale bars: 200 μm (i), 500 μm (a, b, e, g, h) and 1 mm (c, d, f). |
|
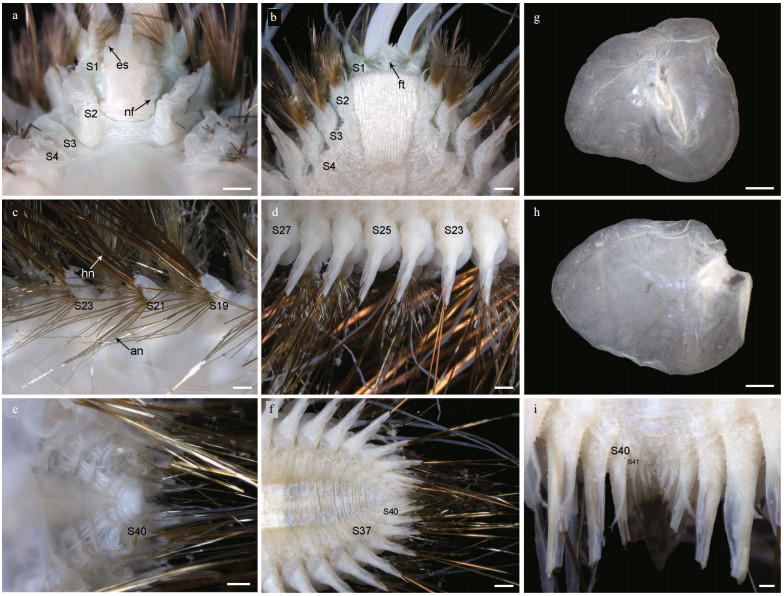
| Fig.4 Parapodia of Laetmonice iocasica sp. nov., holotype a–l. six right parapodia in anterior (left positions) and posterior (right positions) view, notopodium and neuropodium separated in k and l. Abbreviations: S2 referring to segment 2, and so on. Scale bars: 500 μm (a–l). |

|
| Fig.5 Chaetae of Laetmonice iocasica sp. nov., holotype (a, c, d, k, m) and paratypes (b, e–h, j, l: MBM286067; i: MBM286068) a. bipinnate neurochaetae on left 3rd parapodium; b. lowermost capillary notochaetae on right 4th parapodium; c. acicular chaetae on right 1st segment; d. uppermost acicular notochaetae on right 15th parapodium; e. acicular notochaetae on right 4th segment; f. harpoon notochaetae from median segment; g. neurochaetae with basal spur and distal fringe; h. tuberculated spines on left 5th parapodium; i. detail of shaft of tuberculated spines on left 5th parapodium; j. detail of distal parts of harpoon notochaetae (f); k. detail of shafts of harpoon notochaetae from median segment; l, m. detail of distal parts of neurochaetae on left 7th parapodium (l) and right 15th parapodium (m). Scale bars: 100 μm (a–c, e, i, j, l, m), 200 μm (h), 500 μm (g, k), and 1 000 μm (d, f). |
|
Material examined
Holotype: MBM286066, Dive 215, 140°10.8'E, 10°4.9'N, 946 m, 2 June 2019. Paratypes: MBM286067, 1 specimen, Dive 215, 140°11'E, 10°4.8'N, 888 m, 2 June 2019; MBM286068, 1 specimen, Dive 218, Dive 215, 140°14'18"E, 10°07'25"N, 980 m, 6 June 2019.
Diagnosis
Body with 45 segments, maximum length 63.5 mm, maximum width 42.6 mm. Elytra 18 pairs, located on segments 2, 4, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 25, 28, 31, 34, 37, and 40. Dorsum without felt. Ventral surface finely papillated. Palps stout and finely papillated, extending to segment 10. Ocular peduncles without eyes. Nuchal flaps small, not reaching base of ocular peduncles. Facial tubercle with distinct papillae. Bipinnate neurochaetae on segments 2–4. Neurochaetae with basal spur and distal filamentous fringe located from segment 2 to posterior end, usually 3 or 4 per parapodium, a gap between basal spur and distal fringe. Harpoon notochaetae present on elytrigerous segments after segment 5; replaced by 3–7 stout golden brown tuberculated notochaetae on segments 4 and 5. Harpoon notochaetae with shafts covered by fine tubercles, distally with 4 or 5 recurved fangs.
Description (based on type material)
Holotype (Fig. 2a & d), well-preserved with 45 segments, length about 62.2 mm, maximum width 35.1 mm (including chaetae) and 21.2 mm (excluding chaetae). Two paratypes (MBM286067 and MBM286068) both with 45 segments (Fig. 3e, f, i), length 63.4 and 63.5 mm, maximum width 30.4 and 42.6 mm (including chaetae), 21.2 and 25.2 mm (excluding chaetae), respectively. Body ovate to elongate, dorsoventrally flattened (Fig. 2b, c, e, f). Dorsum with no felt covering (Fig. 3c & e). Ventral surface brown to cream-colored, evenly covered by fine papillae (Fig. 3b, f, i). Base of parapodium including position between notopodium and neuropodium also covered by papillae (Fig. 4a–h, k, l).
Prostomium rounded, longer than wide; anterolateral with a pair of cylindrical ocular peduncles (eyestalks), eyes absent (Fig. 3a). Ceratophore of median antenna long, basally articulated (Fig. 3a); style slender with a filamentous tip, 3–4 times as long as prostomium. Palps stout and finely papillated, tapered with fine tips, extending to segment 10. A pair of prominent nuchal flaps, arising from posterolateral part of prostomium, not reaching base of ocular peduncles (Fig. 3a). Facial tubercle located below base of palps, covered by distinct papillae (Fig. 3b).
Elytra 18 pairs, attached to elytrophores on segments 2, 4, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 25, 28, 31, 34, 37, and 40 (Fig. 3a, c, e), completely covering dorsum; elytra soft, opaque, rounded to triangular, without tubercles or papillae on surface and margins (Fig. 3g & h). Dorsal cirri present on segments without elytra; cirrophores short and cylindrical (Fig. 4a, b, g, h), styles slender and smooth with filamentous tips, more than 3 times length of parapodia.
First or tentacular segment with triangular, flattened uniramous parapodia, inserted anterolaterally to prostomium (Figs. 3a, b, 4i, j); 3 tufts of fine, stiff golden acicular chaetae, fanning around margin of parapodia (Figs. 4i & 5c). Two pairs of long dorsal and ventral tentacular cirri, consisting of cylindrical tentaculophores and slender styles with long terminal filaments (Fig. 4i).
Following segments with biramous parapodia. Segment 2–4 with notopodia conical, as long as neuropodia (Fig. 4a–d). Neuropodium conical, with two tiers of neurochaetae (Fig. 4a–d); lower tier with numerous golden bipinnate neurochaetae (Fig. 5a), upper tier with 1–2 stout neurochaetae, with lateral spur on subdistal position and row of numerous filamentous hairs on distal recurved surface (Fig. 5g, l, m). Neuropodia from segment 5 to posterior end elongated, cylindrical with an inflated base (Fig. 4e–h, k, l); 3–4 golden yellow neurochaetae with basal spur and distal filamentous fringe, inserting on distal margin of neuropodia (Fig. 3d & f). Ventral cirri short and smooth, tapered, attached on ventral bases of neuropodia on anterior 4 segments, posteriorly gradually shifting to middle position of ventral surface of neuropodia (Figs. 3b, d, f, i, 4a–h, k, l).
Elytrigerous segments with tuft of 18 or more golden yellow acicular notochaetae on uppermost position (Figs. 3c, 5d, e). Lateral to acicular notochaetae with tuft of usually 6–9 stout golden-brown harpoon notochaetae, inserting on anterior margin of acicular lobes (Figs. 3c, e, 4e, k), directed latero-posteriorly (Fig. 3d–f). Harpoon notochaetae elongated, hollow, basally flattened (Fig. 5f); shafts covered by fine tubercles (Fig. 5k), distally tapering with 4 or 5 recurved fangs on lateral margins (Fig. 5f & j). Harpoon notochaetae replaced on segment 4 and 5 by 3–7 stout golden brown tuberculated notochaetae (Fig. 5h); this notochaetae covered by fine tubercles (Fig. 5i), distally tapering to blunt tips (Fig. 5h). Anterior to harpoon notochaetae or tuberculated notochaetae with small tuft of yellow capillary notochaetae. Notopodial acicula yellow, tapering with blunt tip, projecting from triangular acicular lobe (Fig. 4d, f, l). Ventral to base of notopodium with tuft of short, fine capillary notochaetae (Figs. 4e, f, k, l, 5b).
Cirrigerous segments mainly including three tufts of notochaetae (Fig. 4g). Uppermost position with a fan of golden yellow, smooth acicular notochaetae (Figs. 4a, g, 5c). Ventral to acicular notochaetae with a fan of yellow unidentate notochaetae. Both acicular notochaetae and unidentate notochaetae located on anterior side of acicular lobe (Fig. 4a & g). Acicular notochaetae stouter than unidentate notochaetae (Fig. 4a & g). Posterior to acicular lobes with a fan of capillary notochaetae (Fig. 4b & h).
Etymology
The specific name is derived from the composite of IOCAS (abbreviation of Institute of Oceanology, Chinese Academy of Sciences) and the Latin suffix -icus (belonging to), in celebration of the 70th anniversary of the establishment of the IOCAS in 1950.
Distribution and habitat
Seamounts on the Caroline Ridge in the tropical Western Pacific (888–980-m depth). In situ, the animals crawled slowly on rocky bottoms covered by fine sands (Fig. 2a–c).
Remarks
The number of pairs of elytra is one of the most important characters for separating species of Laetmonice, ranging from 12 pairs in L. batheia Horst, 1916 to 20 pairs in L. producta Grube, 1877 (Hutchings and McRae, 1993). Laetmonice iocasica sp. nov. is a large-sized animal (up to 64 mm) with as many as 45 segments and 18 pairs of elytra. Among all the Laetmonice species, four species have large numbers of segments (> 40 segments) and elytra (18–20 pairs), including L. producta sensu Grube (1877) reported from Kerguelen Islands, L. producta sensu Hutchings and McRae (1993) from the south-eastern Australia, L. producta sensu Imajima (2003) from the Sagami Bay and Suruga Bay in Japan, and L. britannica sensu McIntosh (1900) from Achill Head of Ireland. Laetmonice producta sensu Grube (1877) differs by having 20 pairs of elytra and a pair of eyes, while L. iocasica sp. nov. has 18 pairs of elytra and no eyes. Furthermore, the nuchal flaps are long reaching the base of eyestalk in L. producta sensu Grube (1877) but short in the new species (Table 2). The genetic distance and phylogenetic analyses further supported the separation of the new species and L. producta (Fig. 6; Table 3). Laetmonice iocasica sp. nov. is characterized by the harpoon notochaetae which are covered by fine tubercles on the shafts, while the harpoon notochaetae are smooth or tuberculated in L. producta sensu Hutchings and McRae (1993) and L. producta sensu Imajima (2003). Moreover, the harpoon notochaetae in L. iocasica sp. nov. and L. producta sensu Hutchings and McRae (1993) are replaced on segments 4 and 5 by stout notochaetae without recurved fangs, which is tuberculated in the new species but smooth in the latter (Table 2). The species L. britannica is characterized by reticulate cordate structures on the elytra (Grube, 1877) which are absent in L. iocasica sp. nov.

|
| Fig.6 Bayesian inference (BI) tree reconstructed based on COI sequences of Laetmonice and the other species of Aphroditidae Numbers at the nodes represent ML bootstrap scores (left) / BI posterior probability (right). New species are marked in bold. |
|
Laetmonice producta sensu Hutchings and McRae (1993)
Laetmonice producta. Hutchings and McRae (1993): 333–336, Figs. 45a–f, 46a–j, 59E, Tables 6, 8, 9; Gunton et al. (in press).
Material examined
N2017_V03_014, Australia, Tasmania, Flinders Commonwealth Marine Reserve, 149°6.08'E, 40°27.83'S, CSIRO Four Meter Beam Trawl, 2 298–2 486 m, May 20, 2017.
Description
Large-bodied specimen, body shape elongate, more than twice as long as maximum width. Dorsal felt of fine notochaetae absent, 18 pairs elytra with purple coloration on inner halves. Prostomium with pair of large ocular peduncles, without eye pigment (pigment may be present); facial tubercle well-developed, with long conical papillae; small nuchal flaps present. Palps extending to segment 11, margins finely papillate. Median antenna with ceratophore half length of the prostomium; antennal ceratostyle longer than prostomium, slender, clavate-tipped, three times length of prostomium. Notochaetae of three kinds: ~15 smooth, golden, unidentate acicular chaetae; around ten long, stout, yellow brown harpoon-like chaetae with 3–5 recurved fangs below tips, shafts smooth or tuberculate; tuft of short, fine mud-covered capillary chaetae ventrally. Neurochaetae in two tiers: superior tier of yellow acicular chaetae with basal spur and subdistal fringe of long hairs and bare tips, inferior tier with numerous golden bipinnate neurochaetae. Ventrum covered with small papillae.
Distribution
South-east Australia, from Sandy Cape, Tas. to Broken Bay, New South Wales (457–2 486-m depth).
Remarks
Laetmonice producta displays much morphological variability according to Hutchings and McRae (1993) and has a broad distribution according to McIntosh(1885, 1900), who described several subspecies ("varieties"), ranging from the Azores to Antarctic waters. The variety that occurs in Australian waters is assigned to L. producta by Hutchings and McRae (1993), who examined material from southeast Australia, but these authors suggest a reappraisal of the validity of the subspecies. Live specimens are usually pale, with a longitudinal purple stripe mid-dorsally.
As stated in the remarks above, L. producta sensu Hutchings and McRae (1993) closely resembles L. iocasica sp. nov. in most characters. The two species differ in the details of harpoon notochaetae (tuberculated in L. iocasica vs. tuberculated or smooth in L. producta sensu Hutchings and McRae) and the size of antennae (3–4 length of prostomium vs. 2.5 length of prostomium). Despite the small morphological differences, we suggest they belong to separated species due to their distinct distribution and habitats (Hutchings and Kupriyanova, 2018). All the material cited in Hutchings and McRae (1993) were collected from soft sediments in the southeastern Australia, whereas L. iocasica sp. nov. was sampled on rocky seamounts in the tropical Western Pacific, which are more than 5 000 km apart from the former. In addition, previous studies from off the Australian coast have shown the endemism of the invertebrate seamount fauna (De Forges et al., 2000). Although some widespread species in the deep sea have been reported (Meyer et al., 2008; Georgieva et al., 2015; Guggolz et al., 2020; Zhou et al., 2020), there is no evidence that any species on seamounts can also be found on soft sediments on the seafloor, especially in this case of no obvious ocean current connecting the two locations which are far away.
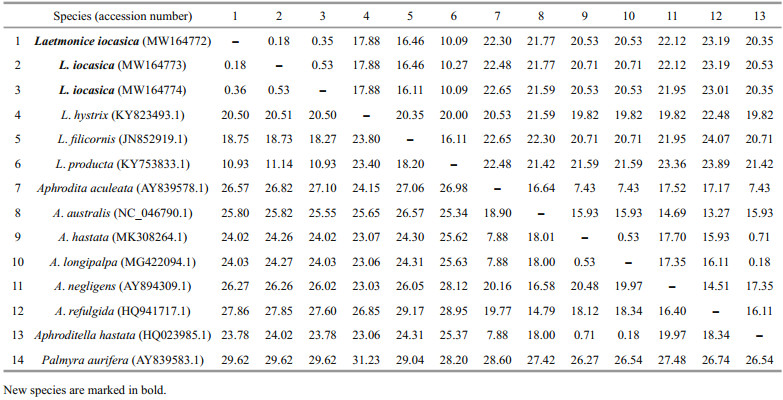
3.2 Barcoding and phylogenetic analysesThree new sequences of COI were obtained from each of the examined specimens. The accession numbers of L. iocasica sp. nov. are MW164772, MW164773, and MW164774, and their lengths are 614 bp. Each gene of 16S (MW288699), 18S (MW288700), 28S (MW288698), and ITS (MW288701) was sequenced successfully from a single specimen and uploaded to GenBank for the supplementary of this study. The alignment sequences of COI comprised 565 nucleotide positions. Pairwise distances based on Kimura 2-parameter and p-distance models were calculated within and among species of Laetmonice and the other genera of Aphroditidae (Table 3). Genetic distances among the species of Laetmonice are 10.93%–23.80% (Kimura 2-parameter) and 10.09%–20.35% (p-distance), while the intraspecific distance within L. iocasica sp. nov. was very low (0.18%–0.53%, based on both models). The genetic distance of Laetmonice producta Grube, 1877 (Table 1) and L. iocasica sp. nov. was in the range of 10.93%–11.14% (Kimura 2-parameter) and 10.09%–10.27% (p-distance), supporting the separation of both species.
Two phylogenetic trees were generated using BI and ML analyses. The topologies were completely consistent, both with high support values in most nodes (ML > 70%, BI > 0.95), thus only the BI tree was displayed with BI and ML support values marked at the nodes (Fig. 6). All available sequences from species of Laetmonice were separated in the phylogenetic trees, and the monophyly of Laetmonice was supported. Laetmonice iocasica sp. nov. and L. producta clustered together with high node support, as consistent with their high morphological similarity.
All the available sequences from species of Aphrodita formed a clade with high support. Genetic distances among the species of Aphrodita are 7.88%–20.16% (Kimura 2-parameter) and 7.43%–17.70% (p-distance), excluding those between A. hastata MK308264.1, A. longipalpa MG422094.1, and Aphroditella hastata HQ023985.1 (Table 3). The sequence information of Aphroditella hastata HQ023985.1 should be revised as Aphrodita hastata HQ023985.1 because the genus Aphroditella has been synonymized with Aphrodita (Pettibone, 1966). The sequence A. longipalpa MG422094.1 was clustered with A. hastata MK308264.1 and Aphroditella hastata HQ023985.1, with Kimura 2-parameter distances ranging from 0.18% to 0.71%, indicating these sequences likely belong to the same species. We suggest that future studies check the type specimens of A. longipalpa and A. hastata and look for precise sequences in subsequent study.
4 DISCUSSIONLaetmonice producta Grube, 1877 was originally described from the area of Kerguelen Islands in the Southern Ocean. Later, McIntosh(1885, 1900) described L. producta dredged off Kerguelen and five varieties of L. producta. These are L. producta var. assimilis McIntosh, 1885 from South of Halifax, Canada, L. producta var. benthaliana McIntosh, 1885 from North Pacific and southern Indian Ocean, L. producta var. britannica McIntosh, 1900 off Achill Head of Ireland, L. producta var. willemoesi McIntosh, 1885 from Azores to the Antarctic Ocean, and north-eastern shores of Australia and New Zealand, and L. producta var. wyvillei McIntosh, 1885 from southern Atlantic Ocean and southern Indian Ocean. Hartman (1965) gathered all the geographical information of the related species and suggested the distribution of the subspecies L. producta producta is restricted from the Kerguelen Islands to the South Georgia Islands and the Antarctic Peninsula. The subspecies was among the most common polychaetes on the Antarctic continental shelf (Parapar et al., 2013).
It is worth mentioning that many of these varieties and subspecies exhibit distinct morphological differences such as the number of segments as well as elytra (Hutchings and McRae, 1993). For example, L. producta var. assimilis, L. producta var. willemoesi and L. producta var. benthaliana generally have 33–35 segments and 15 pairs of elytra, while the others have 43–47 segments and 18–20 pairs of elytra. These differences are sufficient to raise the subspecies to species level. So far, all varieties and subspecies have been raised to species level, including L. producta var. assimilis that was synonymized with L. filicornis (Hartman, 1959; Kongsrud et al., 2013; Read and Fauchald, 2021b). Among these, L. wyvillei and L. benthaliana, were also recorded in the Antarctica (Stiller, 1996).
Laetmonice producta is characterized by 44–47 segments with 20 pairs of elytra, nuchal flaps reaching base of eyestalk, a pair of eyes, palps minutely papillated, and harpoon notochaetae with shafts minutely tuberculated based on the material collected off Kerguelen according to McIntosh (1885) (Table 2). Thus, the populations of L. producta sensu Hutchings and McRae (1993) and L. producta sensu Imajima (2003) likely represent a different species, considering that both populations have 18 pairs of elytra (Table 2). Thus, we agree with Hartman (1965) that Laetmonice producta is probably restrictedly distributed in the Southern Ocean and the Antarctic. The other species related to L. producta, including the species from Japan and Australia, probably belong to a complex of species. A revision of these species is needed based on the examination of the types and voucher specimens.
5 DATA AVAILABILITY STATEMENTSequence data that support the findings of this study have been deposited in the GenBank.
6 ACKNOWLEDGMENTWe are grateful for the crew of the R/V Kexue and the ROV Faxian team, for their effort in sampling the precious material. We also thank Mr. Shaoqing WANG for photographing the fresh specimens onboard.
Barfuss M H J. 2012. Molecular Studies in Bromeliaceae: implications of Plastid and Nuclear DNA Markers for Phylogeny, Biogeography, and Character Evolution with Emphasis on a New Classification of Tillandsioideae. University of Vienna, Vienna.
|
Barnich R, Beuck L, Freiwald A. 2013. Scale worms (Polychaeta: aphroditiformia) associated with cold-water corals in the eastern Gulf of Mexico. Journal of the Marine Biological Association of the United Kingdom, 93(8): 2129-2143.
DOI:10.1017/S002531541300088X |
Barnich R, Fiege D. 2003. The Aphroditoidea (Annelida: Polychaeta) of the Mediterranean Sea. In: Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft. Stuttgart, Schweizerbart. p. 1–167.
|
Brown S, Rouse G, Hutchings P, Colgan D. 1999. Assessing the usefulness of histone H3, U2 snRNA and 28S rDNA in analyses of polychaete relationships. Australian Journal of Zoology, 47(5): 499-516.
DOI:10.1071/ZO99026 |
Carr C M, Hardy S M, Brown T M, Macdonald T A, Hebert P D N. 2011. A tri-oceanic perspective: dNA barcoding reveals geographic structure and cryptic diversity in Canadian Polychaetes. PLoS One, 6(7): e22232.
DOI:10.1371/journal.pone.0022232 |
De Forges B R, Koslow J A, Poore G C B. 2000. Diversity and endemism of the benthic seamount fauna in the southwest Pacific. Nature, 405(6789): 944-947.
DOI:10.1038/35016066 |
Georgieva M N, Wiklund H, Bell J B, Eilertsen M H, Mills R A, Little C T S, Glover A G. 2015. A chemosynthetic weed: the tubeworm Sclerolinum contortum is a bipolar, cosmopolitan species. BMC Evolutionary Biology, 15(1): 280.
DOI:10.1186/s12862-015-0559-y |
Gonzalez B C, Martínez A, Borda E, Iliffe T M, Eibye-Jacobsen D, Worsaae K. 2018. Phylogeny and systematics of Aphroditiformia. Cladistics, 34(3): 225-259.
DOI:10.1111/cla.12202 |
Grube A E. 1877. Anneliden-Ausbeut S.M.S. Gazelle. Die von der Gazelle (Capitain von Schleinitz) mitgebrachten Anneliden, zu denen noch zwei von Dr. Buchholz gesammelte kommen. In: Monatsberichte der Königlich preussischen Akademie der Wissenschaften zu Berlin. p. 509–554.
|
Guggolz T, Meißner K, Schwentner M, Dahlgren T G, Wiklund H, Bonifácio P, Brandt A. 2020. High diversity and pan, oceanic distribution of deep-sea polychaetes: prionospio and Aurospio (Annelida: spionidae) in the Atlantic and Pacific Ocean. Organisms Diversity & Evolution, 20(2): 171-187.
DOI:10.1007/s13127-020-00430-7 |
Gunton L M, Kupriyanova E, Alvestad T et al. An annotated illustrated checklist of the annelid fauna from Australia's eastern lower bathyal and abyssal environment. Zookeys. (in press)
|
Hartman O. 1959. Catalogue of the Polychaetous Annelids of the World. Parts 1 and 2. Allan Hancock Foundation Occasional Paper, 23: 1-628.
|
Hartman O. 1965. Deep-water benthic polychaetous annelids off New England to Bermuda and other North Atlantic areas. Allan Hancock Foundation Publications Occasional Papers, 28: 1-378.
|
Hutchings P A. 2000. Family Aphroditidae. In: Beesley P L, Ross G J B, Glasby C J eds. Polychaetes & Allies: The Southern Synthesis. Fauna of Australia. Vol. 4A Polychaeta, Myzostomida, Pogonophora, Echiura, Sipuncula. CSIRO Publishing: Melbourne. p. 465.
|
Hutchings P, Kupriyanova E. 2018. Cosmopolitan polychaetes, fact or fiction? Personal and historical perspectives. Invertebrate Systematics, 32(1): 1-9.
DOI:10.1071/IS17035 |
Hutchings P, McRae J. 1993. The Aphroditidae (Polychaeta) from Australia, together with a redescription of the Aphroditidae collected during the Siboga Expedition. Records of the Australian Museum, 45(3): 279-363.
DOI:10.3853/j.0067-1975.45.1993.24 |
Imajima M. 1997. Polychaetous annelids of Suruga Bay, Central Japan. National Science Museum Monographs, 12: 149-228.
|
Imajima M. 2003. Polychaetous annelids from Sagami Bay and Sagami Sea collected by the Emperor Showa of Japan and deposited at the Showa Memorial Institute, National Science Museum, Tokyo (II): orders included within the Phyllodocida, Amphinomida, Spintherida and Eunicida. National Science Museum Monographs, 23: 1-221.
|
Kongsrud J A, Budaeva N, Barnich R, Oug E, Bakken T. 2013. Benthic polychaetes from the northern Mid-Atlantic Ridge between the Azores and the Reykjanes Ridge. Marine Biology Research, 9(5-6): 516-546.
DOI:10.1080/17451000.2012.749997 |
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics Analysis across computing platforms. Molecular Biology and Evolution, 35(6): 1547-1549.
DOI:10.1093/molbev/msy096 |
Lamarck J B. 1818. [volume 5 of] Histoire Naturelle des Animaux sans Vertèbres, Préséntant les Caractères Généraux et Particuliers de ces Animaux, leur Distribution, leurs Classes, leurs Familles, leurs Genres, et la Citation des Principales Espèces qui s'y Rapportent; Precedes d'une Introduction Offrant la Determination des Caracteres Essentiels de l'Animal, sa Distinction du Vegetal et Desautres Corps Naturels, Enfin, l'Exposition des Principes Fondamentaux de la Zoologie. Deterville, Paris. p. 612.
|
Linnaeus C. 1758. Systema Naturae per Regna tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, locis. Holmiae, Holmiae, Laurentii.
|
Lobo J, Costa P M, Teixeira M A L, Ferreira M S G, Costa M H, Costa F O. 2013. Enhanced primers for amplification of DNA barcodes from a broad range of marine metazoans. BMC Ecology, 13(1): 34.
DOI:10.1186/1472,6785-13-34 |
Lobo J, Teixeira M A L, Borges L M S, Ferreira M S G, Hollatz C, Gomes P T, Sousa R, Ravara A, Costa M H, Costa F O. 2016. Starting a DNA barcode reference library for shallow water polychaetes from the southern European Atlantic coast. Molecular Ecology Resources, 16(1): 298-313.
DOI:10.1111/1755-0998.12441 |
McIntosh W C. 1885. Report on the Annelida Polychaeta collected by HMS Challenger during the years 1873-76. Challenger Reports, 12: 1-554.
|
McIntosh W C. 1900. A monograph of British Annelids. Volume1, Part 2. Polychaeta Amphinomidae to Sigalionidae. Ray Society of London, 1: 215-442.
|
Medlin L, Elwood H J, Stickel S, Sogin M L. 1998. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene, 71(2): 491-499.
DOI:10.1016/0378-1119(88)90066-2 |
Meyer A, Bleidorn C, Rouse G W, Hausen H. 2008. Morphological and molecular data suggest a cosmopolitan distribution of the polychaete Proscoloplos cygnochaetus Day, 1954 (Annelida, Orbiniidae). Marine Biology, 153(5): 879-889.
DOI:10.1007/s00227-007-0860-4 |
Minh B Q, Nguyen M A T, Von Haeseler A. 2013. Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution, 30(5): 1188-1195.
DOI:10.1093/molbev/mst024 |
Nguyen L T, Schmidt H A, Von Haeseler A, Minh B Q. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution, 32(1): 268-274.
DOI:10.1093/molbev/msu300 |
Norlinder E, Nygren A, Wiklund H, Pleijel F. 2012. Phylogeny of scale-worms (Aphroditiformia, Annelida), assessed from 18SrRNA, 28SrRNA, 16SrRNA, mitochondrial cytochrome c oxidase subunit I (COI), and morphology. Molecular Phylogenetics and Evolution, 65(2): 490-500.
DOI:10.1016/j.ympev.2012.07.002 |
Nygren A, Sundberg P. 2003. Phylogeny and evolution of reproductive modes in Autolytinae (Syllidae, Annelida). Molecular Phylogenetics and Evolution, 29(2): 235-249.
DOI:10.1016/S1055-7903(03)00095-2 |
Parapar J, Moreira J, Gambi M C, Caramelo C. 2013. Morphology and biology of Laetmonice producta producta Grube (Polychaeta: aphroditidae) in the Bellingshausen Sea and Antarctic Peninsula (Southern Ocean, Antarctica). Italian Journal of Zoology, 80(2): 255-272.
DOI:10.1080/11250003.2012.758783 |
Pettibone M H. 1966. Heteraphrodita altoni, a new genus and species of polychaete worm (Polychaeta, Aphroditidae) from deep water off Oregon, and a revision of the Aphroditid genera. Proceedings of the Biological Society of Washington, 79: 95-108.
|
Read G, Fauchald K. 2021a. World Polychaeta database. Laetmonice Kinberg, 1856. Accessed through: world Register of Marine Species. http://www.marinespecies.org/aphia.php?p=taxdetails&id=129196. Accessed on 2020-10-09.
|
Read G, Fauchald K. 2021b. World Polychaeta database. Laetmonice producta Grube, 1877. Accessed through: World Register of Marine Species. http://www.marinespecies.org/aphia.php?p=taxdetails&id=129846. Accessed on 2020-10-09.
|
Ronquist F, Teslenko M, Van Der Mark P, Ayres D L, Darling A, Höhna S, Larget B, Liu L, Suchard M A, Huelsenbeck J P. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61(3): 539-542.
DOI:10.1093/sysbio/sys029 |
Sjölin E, Erséus C, Källersjö M. 2005. Phylogeny of Tubificidae (Annelida, Clitellata) based on mitochondrial and nuclear sequence data. Molecular Phylogenetics and Evolution, 35(2): 431441.
DOI:10.1016/j.ympev.2004.12.018 |
Stiller M. 1996. Distribution and biology of the Aphroditides and Polynoids (Polychaeta) in the eastern Weddell Sea and the Lazarev Sea (Antarctica). Bericht zur Polarforschungen, 185: 1–200, http://hdl.handle.net/10068/255254.
|
Struck T H, Purschke G, Halanych K M. 2005. A scaleless scale worm: molecular evidence for the phylogenetic placement of Pisione remota (Pisionidae, Annelida). Marine Biology Research, 1(4): 243-253.
DOI:10.1080/17451000500261951 |
Vonnemann V, Schrödl M, Klussmann-Kolb A, Wägele H. 2005. Reconstruction of the phylogeny of the Opisthobranchia (Mollusca: gastropoda) by means of 18S and 28S rRNA gene sequences. Journal of Molluscan Studies, 71(2): 113-125.
DOI:10.1093/mollus/eyi014 |
Wang Z X, Li X Q, Xu Z J, Wang Y N, Zheng F R. 2019. First report of the complete mitochondrial genome and phylogenetic analysis of Aphrodita australis (Aphroditidae, Annelida). Mitochondrial DNA Part B, 4(2): 4116-4117.
DOI:10.1080/23802359.2019.1692712 |
Wiklund H, Nygren A, Pleijel F, Sundberg P. 2005. Phylogeny of Aphroditiformia (Polychaeta) based on molecular and morphological data. Molecular Phylogenetics and Evolution, 37(2): 494-502.
DOI:10.1016/j.ympev.2005.07.005 |
Wu X W, Zhan Z F, Xu K D. 2019. Two new and two rarely known species of Branchinotogluma (Annelida: polynoidae) from deep-sea hydrothermal vents of the Manus Back-Arc Basin, with remarks on the diversity and biogeography of vent polynoids. DeepSea Research Part I: Oceanographic Research Papers, 149: 103051.
DOI:10.1016/j.dsr.2019.05.011 |
Zhang D, Gao F L, Jakovlić I, Zou H, Zhang J, Li W X, Wang G T. 2020. PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Molecular Ecology Resources, 20(1): 348-355.
DOI:10.1111/1755-0998.13096 |
Zhang Y J, Sun J, Rouse G W, Wiklund H, Pleijel F, Watanabe H K, Chen C, Qian P Y, Qiu J W. 2018. Phylogeny, evolution and mitochondrial gene order rearrangement in scale worms (Aphroditiformia, Annelida). Molecular Phylogenetics and Evolution, 125: 220-231.
DOI:10.1016/j.ympev.2018.04.002 |
Zhou Y D, Wang Y, Li Y N, Shen C C, Liu Z S, Wang C S. 2020. First report of Osedax in the Indian Ocean indicative of trans-oceanic dispersal through the Southern Ocean. Marine Biodiversity, 50(1): 4.
DOI:10.1007/s12526-019-01034-x |
 2021, Vol. 39
2021, Vol. 39