Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Yang, ZHAN Zifeng, XU Kuidong
- Establishment of Alloptilella splendida gen. et sp. nov. and resurrection of Scytalium veneris (Thomson & Henderson, 1906), two sea pens (Cnidaria: Pennatulacea) from seamounts in the tropical Western Pacific
- Journal of Oceanology and Limnology, 39(5): 1790-1804
- http://dx.doi.org/10.1007/s00343-021-1083-0
Article History
- Received Mar. 12, 2021
- accepted in principle Apr. 15, 2021
- accepted for publication Jun. 23, 2021
2 Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), Zhuhai 519082, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China
The cnidarian order Pennatulacea Verrill, 1865, commonly known as sea pens, are a highly specialized and morphologically distinct group of octocorals, whose colonies are uniquely developed from a single large primary polyp that gives rise to secondary polyps by lateral budding of its body wall (Bayer, 1956; Williams, 1995, 2011). The order comprises 14 families, 37 genera, and more than 200 valid species (Williams, 2011, 2015; García-Cárdenas et al., 2019).
Pennatulacean corals have worldwide geographic and bathymetric distributions (from intertidal to over 6 100 m) and constitute an important component in marine soft-bottom communities, while a few species such as Anthoptilum spp. inhabit rocky substrata by expansion of the proximal end of the peduncle (Williams, 2011; Williams and Alderslade, 2011).
Pennatulaceans have been reported as monophyletic by morphological and molecular analyses (Bayer, 1981; Williams, 1993, 1995, 1997, 2011; McFadden et al., 2006; Daly et al., 2007). The first molecular phylogenetic study focused on Pennatulacea was conducted by Dolan et al. (2013) with an analysis of deep-sea species using ND2 and mtMutS. More recently, Kushida and Reimer (2019) focused on shallow water species in the northwestern Pacific using the same genes. Garciá-Cardenas et al. (2020) investigated pennatulacean phylogeny and divergence times using mtMutS, COI, and 28S rDNA. The data also support the monophyly of Pennatulacea but indicate many pennatulacean families and genera are not monophyletic.
However, the molecular data of most pennatulaceans are lacking, impeding the reconstruction of their phylogenetic relationships. About 86% of the genera and 87% of the species within this order were established before the middle 20th century, many descriptions were based on single and frequently poorly preserved specimens, and some important features such as sclerites were not well illustrated and incompletely described (Kölliker, 1880; Kükenthal, 1915; Hickson, 1916; Williams, 2011; García-Cárdenas et al., 2019). Thus, many taxonomic features that used to define families and genera need to be reevaluated and the classification scheme of Pennatulacea requires revision following both morphological and molecular data.
The family Pennatulidae Ehrenberg, 1834 as well as its type genus Pennatula Linnaeus, 1758 and the family Virgulariidae Verrill, 1868 are representative taxa that need taxonomic revision. For example, García-Cárdenas et al. (2019) resurrected the genus Ptilella Gray, 1870 and transferred three species previously belonging to Pennatula and one newly established species to Ptilella: Pt. bellissima (Fowler, 1888), Pt. grayi García-Cárdenas et al., 2019, Pt. grandis (Ehrenberg, 1834), and Pt. inflata (Kükenthal, 1910). They also suggested the possible re-placement of four species of Pennatula (P. moseleyi, P. naresi, P. murrayi, and P. pearceyi) described by Kölliker (1880) to Ptilella. However, more detailed morphological as well as molecular data are needed to clarify their phylogenetic positions.
During surveys on seamount biodiversity in the tropical Western Pacific, two remarkable colorful sea pens were collected at the water depth of 879 m and 170-291 m, respectively. Based on the morphological and molecular phylogenetic analyses, they are described and illustrated herein as Alloptilella splendida gen. et sp. nov. and Scytalium veneris (Thomson & Henderson, 1906).
2 MATERIAL AND METHOD 2.1 Specimen collectionSpecimens were collected by the Remotely Operated Vehicle (ROV) Faxian (Discovery in Chinese) from two seamounts in the tropical Western Pacific during the cruise of the R/V Kexue (Science in Chinese) in 2014 and 2016. The seamounts located near the Yap Trench (named unofficially as 'Y3') and near the Mariana Trench (named unofficially as 'M2'). The specimens were photographed in situ before being sampled. On board, fresh specimens were photographed as soon as possible; small tissues were cut off and stored at -80 ℃ for DNA extraction. The colonies were fixed in 70% ethanol for morphological studies and permanent preservation. The Specimens are deposited in the Marine Biological Museum of Chinese Academy of Sciences (MBMCAS) at Qingdao, China.
2.2 Morphological examinationMicromorphology were observed and photographed by means of a stereo dissecting microscope (Zeiss SteREO Discovery. V12). Sclerites from autozooid tentacles, pharynx, calyx teeth, calyces/polyp walls, polyp leaves, rachis, and peduncle were isolated respectively by digestion of these tissues in sodium hypochlorite, and then were washed with distilled water and pure ethanol. Sclerites were transferred to carbon double adhesive tape, air-dried and coated for the Scanning Electron Microscope (SEM) to investigate their ultrastructure and size. SEM scans were obtained using Hitachi TM3030Plus SEM at 15 kV and the optimum magnification for each target sclerite. Terminology follows Bayer et al. (1983) and Williams (1995).
2.3 DNA extraction and sequencingGenomic DNA was extracted from the polyp leaves or peduncles using DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) following manufacturer's instructions. The mitochondrial regions mutS homolog (mtMutS) and the 5'-end of the cytochrome coxidase subunit I (COI), and an approximately 750-nt fragment of the 28S nuclear ribosomal gene (28S rDNA) were selected for the genetic analysis. PCR reactions of mtMutS and COI were conducted following Li et al. (2020). PCR reaction for 28S rDNA was performed using primers 28S-Far and 28S-Rar (McFadden and van Ofwegen, 2013) as follows: denaturation at 98 ℃ for 2 min, followed by 30 cycles of denaturation at 98 ℃ for 15 s, annealing at 60 ℃ for 20 s, extension at 72 ℃ for 15 s, and a final extension step at 72 ℃ for 2 min. PCR reactions were performed using I-5TM 2× High-Fidelity Master Mix DNA polymerase (TsingKe Biotech, Beijing, China). PCR products were purified using QIAquick PCR Purification Kit (Qiagen, Hilden, Germany) following manufacturer's instructions, and the sequencing was performed by TsingKe Biological Technology (TsingKe Biotech, Beijing, China).
2.4 Genetic distance and phylogenetic analysesThe phylogenetic reconstructions of concatenated mtMutS, COI, and 28S loci had shown high resolution for the relationship between the similar genera Ptilella Gray, 1870 and Pennatula Linnaeus, 1758 (García-Cárdenas et al., 2019, 2020), and thus we selected the concatenated marker for the present analysis. The sequence database mainly followed García-Cárdenas et al. (2019) plus six Virgulariid species and the newly sequenced specimens (Table 1). The sequences were aligned using MAFFT v.7 (Katoh and Standley, 2013) with the G-INS-i and Q-INS-i algorithms for the mitochondrial genes and 28S rDNA regions, respectively. The concatenated sequence alignment was combined using BioEdit v7.0.5 (Hall, 1999). The alignments of mtMutS and the concatenated mtMutS-COI-28S had lengths of 686 and 1 981 positions, respectively. Genetic distances of mtMutS among species were calculated with MEGA 6.0 using Kimura two-parameter model (Tamura et al., 2013).
Model selection and Maximum likelihood (ML) analyses were carried out using PhyML ver. 3.0 (Guindon et al., 2010) on the online ATGC bioinformatic platform (http://www.atgc-montpellier.fr/phyml/). The best-fit model GTR+G+I for the concatenated marker were selected by SMS (Lefort et al., 2017) with the Akaike information criterion in PhyML ver. 3.0. Node support for the ML trees came from a majority-rule consensus tree of 1 000 bootstrap replicates. For the ML bootstraps, we consider values < 70% as low, 70%-94% as moderate, and ≥95% as high, following Hillis and Bull (1993). Bayesian inference (BI) analysis was carried out using MrBayes ver. 3.2.3 (Ronquist and Huelsenbeck, 2003) on the CIPRES Science Gateway. Posterior probability was estimated using four chains running 10 000 000 generations sampling every 1 000 generations. The first 25% of sampled trees were considered burn-in trees. Convergence was assessed by checking the standard deviation of partition frequencies (< 0.01), the potential scale reduction factor (~1.00), and the plots of log-likelihood values (no obvious trend was observed over time) using Tracer ver. 1.5 (Rambaut and Drummond, 2007). For the Bayesian posterior probabilities, we consider values < 0.95 as low and ≥0.95 as high, following Alfaro et al. (2003).
3 RESULT 3.1 SystematicsClass Anthozoa Ehrenberg, 1834
Subclass Octocorallia Haeckel, 1866
Order Pennatulacea Verrill, 1865
Family Pennatulidae Ehrenberg, 1834
Genus Alloptilella gen. nov.
Diagnosis: Colonies pinnate. Axis circular in cross section, present throughout length of colony. Rachis bilaterally symmetrical, with a central dorsal track naked of zooids. Rachis-peduncle limit with a distinct thickening or swelling that forms an edged ring at the thickest point. Polyp leaves large and conspicuous, deltoid, alternately disposed and inserted obliquely, extending ventrally upward. Autozooids in a row along the ventral edge of polyp leaves. Anthocodiae retractile into permanent spiculiferous calyces. Calyces tubular, with up to eight terminal teeth. Siphonozooids on lateral sides of rachis and between polyp leaves. Mesozooids distributed on lateral sides of rachis near dorsal base of polyp leaves. Three-flanged needles on autozooid tentacles, calyces, polyp leaves and rachis, inconspicuous three-flanged rods on peduncle.
Type species: Alloptilella splendida gen. et sp. nov.
Etymology: Composite of the Greek prefix allo-(another, different) and the generic name Ptilella. Gender feminine.
Alloptilella splendida gen. et sp. nov. (Figs. 1 & 2)

|
| Fig.1 External morphology of Alloptilella splendida gen. et sp. nov., holotype a. newly collected colony, photographed by Wei JIANG; b. preserved colony; c. colony in situ; d. being collected by ROV; e. lateral view of autozooids; f. oral view of autozooids showing their arrangement; g. proximal dorsal edge of a polyp leaf and neighboring lateral side of rachis, arrows indicate mesozooids; h. siphonozooids and their arrangement, arrows indicate mesozooids. Scale bars: 5 cm (a, b) and 1 mm (e-h). |

|
| Fig.2 Sclerites of Alloptilella splendida gen. et sp. nov. in different tissues of holotype a. tentacles; b. calyx teeth of autozooids; c. autozooid calyces; d. polyp leaves; e. cortex of rachis containing siphonozooids; f. cortex of peduncle; g. pharynx of autozooids. Scale bars: 100 μm (b-e) and 20 μm (a, f, g). |
Pennatula moseleyi. Xu et al., 2020: 115 (photo).
Material examined: Holotype: MBM286413, collected from Y3 seamount at the station FX-Dive 16 (8°51.80'N, 137°44.58'E) in water depth of 879 m on December 15, 2014, foraminiferal ooze bottom.
Description: Colony and size: Colony pinnate, slender and straight, buried in bottom by peduncle in situ; measuring 550 mm in length in preservation (Fig. 1a-d). Axis extending throughout length of the colony. Axis circular in cross section, off-white in color, maximum diameter 4.4 mm below rachis-peduncle limit 14 mm. Peduncle damaged in collection, 90 mm in length left (16% of total length) including a thickening (10 mm in width). Rachis bilaterally symmetrical, 460 mm in length (84% of total length), usually 11-18 mm in width, sub-equal excepting the lowest part. Rachis with a distinctive naked central dorsal track and a hidden ventral track occupied by insertions of polyp leaves. Rachis-peduncle limit with a distinct swelling that forms an edged ring at the widest point (Fig. 1a-b). Rachis with 65 pairs of polyp leaves, with the lowest 7 pairs rudimentary, averaging 14.1 pairs per 100-mm long. Polyp leaves inserted obliquely, alternately disposed, extending ventrally upward. Fully developed polyp leaves deltoid in lateral view, with the bases 18 mm in maximum length, the ventral edges curved with maximum length 23 mm, and dorsal edges slightly shorter than ventral edges.
Polyps: Autozooids arranged in one row along the ventral edge of polyp leaves (indicated by arrangement of gastrovascular cavities by making transversal sections of the polyp leaves), they sometimes crowdedly and alternately arranged that appear to be placed in two rows; maximum 26 autozooids per developed polyp leaf (Fig. 1e-f). Anthocodiae retractile into permanent spiculiferous calyces. Calyces tubular, spiculate, armed with up to 8 strongly projecting teeth, and up to 4 mm in length and 2 mm in width (Fig. 1e-f). Mesozooids distributed on the lateral side of rachis near the dorsal base of polyp leaves, spiculiferous, armed with yellowish sclerites, approximately 0.5 mm in height and 1.0 mm in width (Fig. 1g). Siphonozooids minute but conspicuous in light microscope, approximately 0.2 mm in height and 0.3 mm in width, mostly conical with converging reddish sclerites forming a pointed apex; arranged in 1-10 rows along the axillae of polyp leaves to the latero-dorsal sides of the rachis between polyp leaves (Fig. 1h). Dorsal track devoid of zooids throughout length of rachis.
Sclerites: Aboral sides of autozooid tentacle rachis with small three-flanged needles, approximately 116- 176 μm in length (Fig. 2a). Calicular teeth composed of large three-flanged needles, 500-1 200 μm in length (Fig. 2b). Sclerites in calyces excluding teeth also three-flanged needles, 491-731 μm in length (Fig. 2c). Polyp leaves contained numerous three-flanged needles, 424-669 μm in length (Fig. 2d). Three-flanged needles from cortex of rachis containing siphonozooids, 178-637-μm long (Fig. 2e). Peduncle with short three-flanged rods, 70-83 μm in length (Fig. 2f). Pharynx of autozooids with small three-flanged needles, 39-54 μm in length (Fig. 2g).
Color: Rachis generally red with yellow calyces. Peduncle yellowish. Anthocodiae mainly white with red lines along aboral side of each tentacle rachis and extending to autozooid wall due to the presence of colored sclerites (Fig. 1). Needles from calicular teeth and upper calyces yellowish, needles from tentacles, lower calyces, polyp leaves and rachis reddish, and rods from peduncle translucent to whitish.
Etymology: The Latin adjective splendidus (splendid) refers to the beautiful reddish and golden color of the species.
Distribution: Known only from the seamount Y3 near the Yap Trench in the Tropical Western Pacific, where the water depth was 879 m, water temperature about 4.95 ℃, and salinity about 34.5. In situ, the species buried in foraminiferal ooze.
Family Virgulariidae Verrill, 1868 Genus Scytalium Herklots, 1858
Scytalium veneris (Thomson & Henderson, 1906) (Figs. 3-5)

|
| Fig.3 Morphology of Scytalium veneris (Thomson & Henderson, 1906) a. dorsal view of colony in situ; b. ventral view of colony in situ; c. lateral view of colony in situ; d. preserved colony (MBM286416); e. three preserved colonies (MBM286417); f. a newly collected colony (MBM286417), potographed by Xuwen WU; g. live colonies in aggregation, laser dots spaced at 33 cm used for measuring dimensions; h. cross section of axis at upper peduncle. Scale bars: 5 cm (d-f) and 0.5 mm (h). |
|
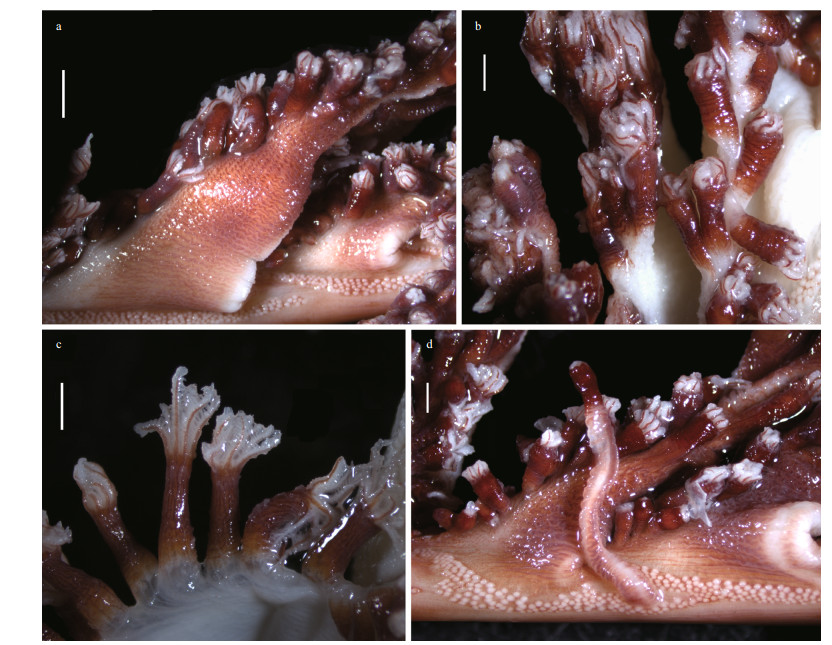
| Fig.4 Polyp leaves and polyps of Scytalium veneris (Thomson & Henderson, 1906) a. polyp leaves and siphonozooids at the bases of polyp leaves; b, c. autozooids in preservation; d. one elongated autozooid and adjacent siphonozooids. Scale bars: 2 mm (a) and 1 mm (b-d). |
|
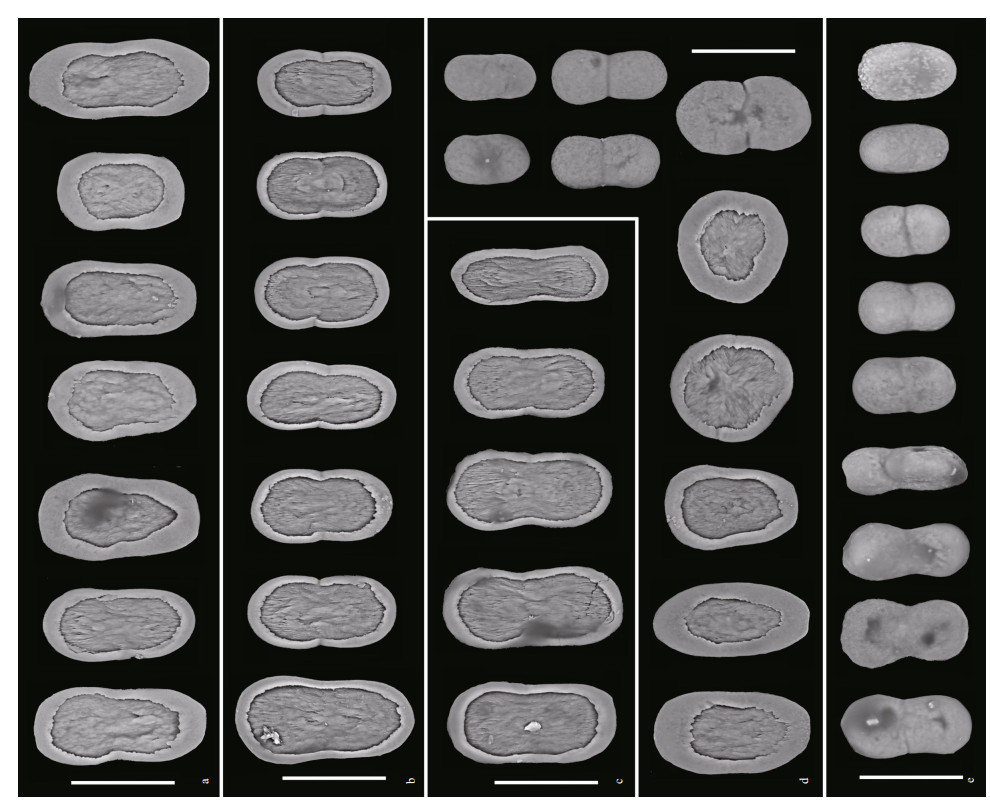
| Fig.5 Sclerites of Scytalium veneris (Thomson & Henderson, 1906) in different tissues a. tentacles; b. polyp walls; c. polyp leaves; d. cortex of rachis; e: cortex of peduncle. Scale bars: 30 μm. |
Pennatula veneris Thomson & Henderson, 1906: 115-116, Pl. Ⅷ. Fig. 8.
Scytalium sarsii: Kükenthal, 1915: 66 (in part).
Scytalium veneris (Thomson & Henderson, 1906): Hickson, 1916: 205.
Scytalium cf. sarsii. Xu et al., 2020: 116-117 (photo).
Material examined: MBM286416, 1 specimen, collected from M2 seamount at the station FX-Dive 58 (11°19.46'N, 139°19.60'E) with the water depth of 249 m on 15 March 2016, buried in debris of Halimeda sp. (Chlorophyta: Ulvophyceae: Halimedaceae); MBM286417, 3 specimens, collected from M2 seamount at the station FX-Dive 67 (11°19.47'N, 139°18.51'E) with the water depth of 275-291 m on 24 March 2016, buried in debris of Halimeda sp.
Description: Colony and size: Colonies feather-like in life; preserved specimens elongate, 288- 337 mm in length (Fig. 3a-g). Axis extending throughout length of the colony, circular in cross section, maximum diameter 1.5-1.8 mm at lower-most rachis naked of polyp leaves (Fig. 3h). Peduncle 41-62 mm in length, with a bulb at uppermost. Rachis 245-275 mm in length, occupying 82%-86% of entire length; tapering upwards, with maximum width including polyp leaves approximately 16 mm, and maximum diameter excluding polyp leaves 3 mm (Fig. 3d-e). Rachis-peduncle limit with a distinct swelling forming an edged ring at the thickest point. Rachis with 28-36 pairs of alternately disposed polyp leaves, averaging 11.3-14.2 pairs per 100-mm long. Polyp leaves triangular in lateral view, with proximal bases attached to rachis usually 10-14 mm in length, the ventral margin bearing polyps usually 10-20 mm in length, the dorsal margin free of polyps usually 15-25 mm in length (Fig. 4). Each developed leaf usually bearing 32-40 autozooids arranged in one row.
Polyps: Autozooids tubular, usually 3-4 mm in length and approximately 1 mm in diameter; a few autozooids reaching 9 mm in length occurred on dorsal sides of a polyp leaf or rachis (Fig. 4). Autozooid walls not forming rigid calyces; tentacles contractile, non-retractile. Siphonozooids conspicuous, longitudinally placed in lateral sides of the rachis below the proximal bases of polyp leaves (Fig. 4a & d). Siphonozooids rounded, approximately 0.1 mm in height and 0.2 mm in diameter. Mesozooids absent.
Sclerites: Autozooid tentacles, polyp walls, and polyp leaves containing almost dumbbell-shaped plates, those from tentacles mostly ranging 37-52 μm in length and 21-26 μm in maximum width, those from polyp walls ranging 38-48 μm in length and 20-26 μm in width, those from polyp leaves ranging 44-48 μm in length and 16-24 μm in width (Fig. 5a-c). Cortex of rachis containing oval-shaped to rounded plates ranging 32-45 μm in length and 21-32 μm in width, and elliptical to dumbbell-shaped rods, ranging 24-39 μm in length and 13-22 μm in width (Fig. 5d). Cortex of peduncle containing dumbbell-shaped to oval-shaped rods, mostly ranging 26-38 μm in length and 14-20 μm in width (Fig. 5e). Plates and rods with broadly-rounded ends, and mostly constricted at middles.
Color: Peduncle brown reddish, rachis and polyp leaves dark brown reddish to off-white (Fig. 3). Autozooids mainly dark brown reddish, tentacles white with a red line in aboral side of each rachis (Fig. 4). Axis off-white. Sclerites reddish. Plates from tentacles, polyp walls, polyp leaves, and cortex of rachis reddish; rods from cortex of rachis and penuncle reddish to transparent.
Distribution: Previously known only from the Indian Ocean at the water depth of 183 m (Thomson and Henderson, 1906). Newly recorded on M2 seamount near the Mariana Trench in the Tropical Western Pacific, where the water depths were 170- 291 m; the water temperature was about 11.6 ℃ and salinity about 34.4 at water depth of 249 m. In situ, the species buried in debris of Halimeda sp., occurred in large quantity, and could reach about 40 colonies per square meter on the seamount.
3.2 Genetic distance and phylogenetic analysesThe new sequences have been deposited in GenBank (Table 1). The mtMutS genetic distances between the uncertain species Pennatula cf. inflata MK919666 and Ptilella were in range of 0-0.34%, indicating the species probably belong to Ptilella, which supports the transfer of Pennatula inflata to Ptilella suggested by García-Cárdenas et al. (2019) (Supplementary Table S1). The mtMutS genetic distances between Alloptilella splendida gen. et sp. nov. and Pennatula sp. 2 DQ302870 reported from the Tasman Sea was 0.17%, suggesting the latter specimen is likely to belong to Alloptilella gen. nov. (Supplementary Table S1). Excluding these two undetermined, poorly studied specimens (Ptilella cf. inflata MK919666 and Pennatula sp. 2 DQ302870), the mtMutS genetic distances between Alloptilella gen. nov. and Ptilella ranged from 3.17%-3.53%., those between Alloptilella gen. nov. and Pennatula ranged from 4.28%-6.14%, and those between the new genus and Scytalium ranged from 5.01%-5.41% (Supplementary Table S1). By contrast, the interspecific genetic distances of mtMutS within Ptilella, Pennatula, and Scytalium are no more than 2.63% (Supplementary Table S1), supporting Alloptilella as a new genus.
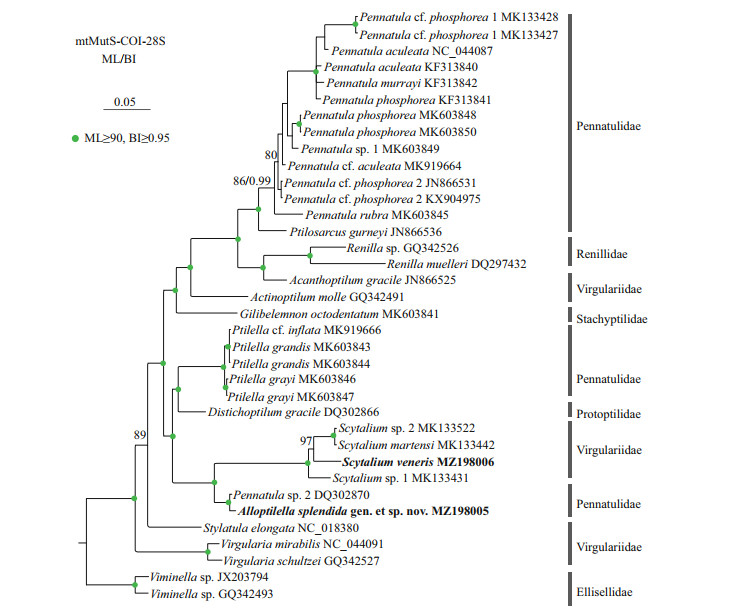
The ML tree is nearly identical with the BI tree in topology, and thus only the former with both support values is shown (Fig. 6). In the trees, Scytalium veneris clustered with the group including S. martensi, Scytalium sp. 1, and Scytalium sp. 2, and all the Scytalium species formed a monophyletic clade with high support. Instead of clustering with Pennatula or Ptilella, Alloptilella splendida gen. et sp. nov. formed a sister clade with Scytalium with high support. Both the families Virgulariidae and Pennatulidae are polyphyletic.
|
| Fig.6 Maximum likelihood (ML) tree constructed by the concatenated mtMutS-COI-28S showing phylogenetic relationships of Alloptilella splendida gen. et sp. nov., Ptilella, Pennatula, and related genera Node support is as follows: ML bootstrap /posterior probability of Bayesian inference (BI). The ML bootstrap < 70% or BI posterior probability < 0.90 is not shown. Newly sequenced species are in bold. The GenBank accession numbers of the mtMutS sequences were listed next to the species names. |
Ptilella is a genus recently resurrected by García-Cárdenas et al. (2019) to contain a new species and three former species of Pennatula having the following features: 1) a dorsal track free of zooids; 2) autozooids arranged in many oblique rows each usually composed of 3 or 4 polyps on the ventral edge of polyp leaves; 3) polyp leaves obliquely inserted and ventrally upward extended on the rachis; 4) mesozooids on the rachis and the proximal 2/3 part of the dorsal edge of polyp leaves; and 5) a distinct thickening at rachis-peduncle limit. Alloptilella splendida gen. et sp. nov. matches the definition of Ptilella Gray, 1870 in most main features, but differs in the arrangement of autozooids (in many oblique rows vs. in a single row) and the location of mesozooids (on the dorsal side of rachis near the dorsal base of polyp leaves vs. on both the rachis and the proximal 2/3 part of the dorsal edge of polyp leaves). The arrangement of autozooids and location of mesozooids are two important features utilized by García-Cárdenas et al. (2019) to differentiate Ptilella from Pennatula, in which the autozooids are arranged in a single row along the ventral edge of polyp leaves and the mesozooids are located on the rachis or the basal dorsal edge of the polyp leaves. Therefore, we establish Alloptilella gen. nov. to accommodate Alloptilella splendida gen. et sp. nov. The generic separation is well supported by their genetic distances and the molecular phylogenetic trees constructed by the concatenated mtMutS-COI-28S. Nonetheless, the familial assignment of the new genus is difficult. In the phylogenetic trees, Alloptilella was clustered with the genus Scytalium of the family Virgulariidae (Williams, 1995). However, both the morphology and genetic distance indicate that Alloptilella is more closely related to Ptilella, a genus of the family Pennatulidae (Supplementary Table S1). Thus, we tentatively assign the new genus to the polyphyletic family Pennatulidae.
García-Cárdenas et al. (2019) indicated four species of Pennatula including P. moseleyi and P. naresi described by Kölliker (1880) might belong to Ptilella but suggested more improved morphological and molecular information. Therefore, the generic assignment of these four species is doubtful and we still leave them in Pennatula tentatively. Alloptilella splendida gen. et sp. nov. was commonly misidentified as Pennatula moseleyi due to their resemblance in overall appearance, especially the remarkable reddish rachis and yellowish calyces (Xu et al., 2020: 115; the water depth was also mistakenly recorded). By detailed comparison, we found that except the generic differences, the polyp leaves of Alloptilella splendida gen. et sp. nov. are less crowded than those of P. moseleyi (averaging 14.1 pairs vs. 18.8 pairs per 100 mm). Alloptilella splendida gen. et sp. nov. is very similar to Pennatula naresi in overall appearance, but differs by the location of mesozooids (the ventral zooids in Kölliker (1880:3)) and the color of sclerites surrounding siphonozooids. The mesozooids of Alloptilella splendida gen. et sp. nov. are just located on the dorsal side of rachis near the dorsal base of polyp leaves, while those of P. naresi are located on the proximal dorsal edge of polyp leaves and continuing along the boundary line of dorsal and lateral side of rachis to the dorsal base of neighboring polyp leaves (Imahara et al., 2014; García-Cárdenas et al., 2019). The sclerites surrounding the siphonozooids of Alloptilella splendida gen. et sp. nov. are reddish, while those of P. naresi are yellowish (Kölliker, 1880; Nutting, 1912; Imahara et al., 2014). Although Imahara et al. (2014), Hickson (1916), and García-Cárdenas et al. (2019) mentioned the autozooids of P. naresi are arranged in two rows, it is probably that they are actually closely arranged in one row but appear as two rows, as in Alloptilella splendida gen. et sp. nov.
4.2 Resurrection of Scytalium veneris (Thomson & Henderson, 1906)Scytalium Herklots, 1858 is characterized by a bilateral symmetrical rachis, small oval-shaped plates in the autozooids, polyp leaves and rachis, and oval-shaped rods in the rachis and peduncle. It is a well defined genus, though the validity of some species has been disputed (e.g. Hickson, 1916; Williams, 1995). Seven nominal species collected from water depths of 18-489 m have been assigned to Scytalium, all of them are considered here as valid: Scytalium balssi Hickson, 1916 from the Timor sea; Scytalium herklotsi López-González, 2021 from the Caribbean Sea; S. martensi Kölliker, 1870, S. sarsii Herklots, 1858 and S. splendens (Thomson & Henderson, 1906) from the Indo-West Pacific; S. tentaculatum Kölliker, 1880 from the sea area of Philippines; S. veneris (Thomson & Henderson, 1906) from the Indian Ocean (Herklots, 1858; Kölliker, 1870, 1880; Thomson and Henderson, 1906; Hickson, 1916; Williams, 1995; Tang, 2008; Imahara et al., 2014; Imahara and Namikawa, 2018; López-González, 2021).
Our specimens match well with Scytalium veneris (Thomson & Henderson, 1906) in all the diagnostic characters, including a circular axis in cross-section, the absence of the digitiform process, the mumber of polyp leaves (56-72 vs. 47-65), the sizes of polyp leaves (length 15-25 mm vs. 16-26.5 mm), the number and arrangement of autozooids in developed leaves (32-40 in one row vs. 30-36 in one row), the size of autozooids (length both up to 9 mm), the arrangement of siphonozooids as long narrow bands, and the sizes of plates (length 32-52 μm vs. 40-45 μm). Scytalium veneris was considered as a junior synonym of S. sarsii by Kükenthal (1915). However, S. veneris differs distinctly from S. sarsii by a circular axis in cross-section (vs. quadrangular), much larger polyp leaves (length of ventral edges 10-20 mm vs. 4-8 mm), the number and arrangement of autozooids in developed leaves (32-40 in one row vs. 18-25 in 2 or 3 rows), the size of autozooids (length usually 3-4 mm and up to 9 mm vs. no more than 0.6 mm), and mostly larger plates (length 32-52 μm vs. maximum 33 μm) (Herklots, 1858; Kölliker, 1870, 1880; Thomson and Henderson, 1906; Hickson, 1916).
Scytalium veneris mostly resembles S. splendens in the colony shape (both stout and robust rather than whip-like) and the size of polyp leaves (length 15- 26.5 mm vs. 23-25 mm), but differs from the latter by the presence of whip-like autozooids, the number of autozooids in each developed polyp leaf (30-40 vs. 50-60), the red bands of sclerites in the aboral sides of tentacles (vs. absence), the arrangement of siphonozooids, and the color of polyp leaves (Thomson and Henderson, 1906; Hickson, 1916; Imahara et al., 2014).
Scytalium was assigned to the family Pennatulidae by Hickson (1916), then to the family Virgulariidae by Williams (1995). Our molecular phylogenetic analysis indicates that Scytalium is a monophyletic group. Nonetheless, the affiliation of Scytalium in Virgulariidae is pending, as already questioned by Kushida and Reimer (2019), because the family is polyphyletic. More molecular data are needed to clarify the phylogenetic positions of pennatulacean families and genera and to evaluate the significance of taxonomic features used in discriminating different taxa.
5 CONCLUSIONTwo pennatulaceans collected from seamounts in the tropical Western Pacific are described and illustrated as Alloptilella splendida gen. et sp. nov. and Scytalium veneris (Thomson & Henderson, 1906). The arrangement of autozooids and the location of mesozooids are considered as two main generic features separating the new genus Alloptilella gen. nov. from the morphologically similar genus Ptilella. The generic separation as well as the importance of the location of mesozooids in generic delimitation is demonstrated by their genetic distances and the molecular phylogenetic analyses. Alloptilella splendida gen. et sp. nov. mostly resembles Pennatula naresi, but differs by the location of mesozooids and the reddish color of sclerites surrounding siphonozooids. Scytalium veneris (Thomson & Henderson, 1906) is resurrected by recognizing the distinctive differences between it and Scytalium sarsii Herklots, 1858. This is the first record of Scytalium veneris outside its type locality, and the molecular phylogenetic analysis indicates that Scytalium is a monophyletic group. Both the families Pennatulidae and Virgulariidae as well as the genus Pennatula are polyphyletic; more morphological and molecular data are needed to clarify the phylogenetic positions of these pennatulacean families and related genera.
6 DATA AVAILABILITY STATEMENTSequence data analyzed during this study has been deposited in the GenBank.
7 ACKNOWLEDGMENTWe appreciate the crew of R/V Kexue and ROV Faxian, Center for Ocean Mega-Science, Chinese Academy of Sciences, for their assistance in sample and data collection. We thank our colleagues Drs. Wei JIANG and Xuwen WU for photographing freshly collected specimens on board and Ms. Ting LV for extracting genomic DNA of studied specimens. We also appreciate Dr. Pablo J. López-González (Universidad de Sevilla, Spain) providing us comments and literature.
Electronic supplementary materialSupplementary material (Supplementary Table S1) is available in the online version of this article at https://doi.org/10.1007/s00343-021-1083-0.
Alfaro M E, Zoller S, Lutzoni F. 2003. Bayes or Bootstrap? A simulation study comparing the performance of Bayesian Markov chain Monte Carlo sampling and bootstrapping in assessing phylogenetic confidence. Molecular Biology and Evolution, 20(2): 255-266.
DOI:10.1093/molbev/msg028 |
Bayer F M, Grasshoff M, Verseveldt J. 1983. Illustrated Trilingual Glossary of Morphological and Anatomical Terms Applied to Octocorallia. E. J. Brill/Dr. W. Backhuys, Leiden. 75p.
|
Bayer F M. 1956. Octocorallia. In: Moore R C ed. Treatise on Invertebrate Paleontology. Part F. Coelenterata. University of Kansas Press, Lawrence. p. 166-231.
|
Bayer F M. 1981. Key to the genera of Octocorallia exclusive of Pennatulacea (Coelenterata: Anthozoa), with diagnoses of new taxa. Proceedings of the Biological Society of Washington, 94(3): 902-947.
|
Daly M, Brugler M R, Cartwright P, Collins A G, Dawson M N, Fautin D G, France S C, McFadden C S, Opresko D M, Rodriguez E, Romano S L, Stake J L. 2007. The phylum Cnidaria: a review of phylogenetic patterns and diversity 300 years after Linnaeus. In: Zhang Z Q, Shear W A eds. Linnaeus Tercentenary: Progress in Invertebrate Taxonomy. Zootaxa, 1688. Magnolia Press, Auckland, New Zealand. p. 127-182, http://hdl.handle.net/1808/13641.
|
Dolan E, Tyler P A, Yesson C, Rogers A D. 2013. Phylogeny and systematics of deep-sea sea pens (Anthozoa: Octocorallia: Pennatulacea). Molecular Phylogenetics and Evolution, 69(3): 610-618.
DOI:10.1016/j.ympev.2013.07.018 |
Ehrenberg C G. 1834. Die Corallenthiere Des Rothen Meeres. Abhandlung der Königlichen Akademie der Wissenschaften, Berlin. 156p.
|
Fowler G H. 1888. On a new Penmatula from the Bahanias. Proceedings of the Zoological Society of London, 56(1): 135-140.
DOI:10.1111/j.1469-7998.1888.tb06690.x |
García-Cárdenas F J, Drewery J, López-González P J. 2019. Resurrection of the sea pen genus Ptilella Gray, 1870 and description of Ptilella grayi n. sp. from the NE Atlantic(Octocorallia: Pennatulacea). Scientia Marina, 83(3): 261-276.
DOI:10.3989/scimar.04845.26A |
Garciá-Cardenas F J, Núñez-Flores M, López-González P J. 2020. Molecular phylogeny and divergence time estimates in pennatulaceans (Cnidaria: Octocorallia: Pennatulacea). Scientia Marina, 84(4): 317-330.
DOI:10.3989/scimar.05067.28A |
Gray J E. 1870. Catalogue of Sea-Pens or Pennatulariidae in the Collection of the British Museum. British Museum, London. 40p.
|
Guindon S, Dufayard J F, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology, 59(3): 307-321.
DOI:10.1093/sysbio/syq010 |
Haeckel E. 1866. Generelle Morphologie der Organismen. Allgemeine Grundzüge der Organischen FormenWissenschaft, Mechanisch Begründet Durch die von Charles Darwin Reformierte Descendenz-Theorie. Georg Reimer, Berlin.
|
Hall T A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41: 95-98.
|
Herklots J A. 1858. Notices pour servir à l'étude des Polypiers nageurs ou Pennatulides. Bijdragen tot de Dierkunde, 7(1): 1-30.
DOI:10.1163/26660644-00701001 |
Hickson S J. 1916. The Pennatulacea of the Siboga expedition, with a general survey of the order. Siboga Expeditie Monographs, 14(Livr. 77): 1-265.
|
Hillis D M, Bull J J. 1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology, 42(2): 182-192.
DOI:10.1093/sysbio/42.2.182 |
Imahara Y, Iwase F, Namikawa H. 2014. The Octocorals of Sagami Bay. Tokai University Press, Hadano. 398p.
|
Imahara Y, Namikawa H. 2018. Preliminary report on the octocorals (Cnidaria: Anthozoa: Octocorallia) from the Ogasawara Islands. Memoirs of National Museum Nature and Science Tokyo, 52: 65-94.
|
Katoh K, Standley D M. 2013. MAFFT Multiple Sequence Alignment Software version 7:improvements in performance and usability. Molecular Biology and Evolution, 30(4): 772-780.
DOI:10.1093/molbev/mst010 |
Kölliker R A. 1870. Anatomisch-systematische Beschreibung der Alcyonarien. Erster Abtheilung: Die Pennatuliden. Abhandlungen Senckenbergische Naturforschende Gesellschaft, 7: 487-602, pls. 11-17.
|
Kölliker R A. 1880. Report on the Pennatulida dredged by H. M. S. Challenger during the years 1873-1876. Report of the Scientific Results of the Voyage of H. M. S. Challenger during the Years 1873-1876. Zoology, 1(2): 1-41, pls. 1-11.
|
Kükenthal W. 1910. Pennatuliden der Deutschen tiefseeexpedition. Zoologischer Anzeiger, 36(2-3): 51-58.
|
Kükenthal W. 1915. Pennatularia. Das Tierreich. Verlag von R. Friedländer und Sohn, Berlin. 132p.
|
Kushida Y, Reimer J D. 2019. Molecular phylogeny and diversity of sea pens (Cnidaria: Octocorallia: Pennatulacea) with a focus on shallow water species of the northwestern Pacific Ocean. Molecular Phylogenetics and Evolution, 131: 233-244.
DOI:10.1016/j.ympev.2018.10.032 |
Lefort V, Longueville J E, Gascuel O. 2017. SMS: smart model selection in PhyML. Molecular Biology and Evolution, 34(9): 2 422-2 424.
DOI:10.1093/molbev/msx149 |
Li Y, Zhan Z F, Xu K D. 2020. Morphology and molecular phylogenetic analysis of deep-sea purple gorgonians(Octocorallia: Victorgorgiidae) from seamounts in the tropical Western Pacific, with description of three new species. Frontiers in Marine Science, 7: 701.
DOI:10.3389/fmars.2020.00701 |
Linnaeus C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. In: Tomus I ed. Decima, Reformata. Laurentii Salvii, Holmiae. 824p.
|
López-González P J. 2021. Scytalium herklotsi sp. nov. (Anthozoa, Octocorallia, Pennatulacea), the first Atlantic species in the genus Scytalium Herklots, 1858. Marine Biodiversity, 51: 62.
DOI:10.1007/s12526-021-01200-0 |
McFadden C S, France S C, Sánchez J A, Alderslade P. 2006. A molecular phylogenetic analysis of the Octocorallia(Cnidaria: Anthozoa) based on mitochondrial proteincoding sequences. Molecular Phylogenetics and Evolution, 41(3): 513-527.
DOI:10.1016/j.ympev.2006.06.010 |
McFadden C S, van Ofwegen L P. 2013. A second, cryptic species of the soft coral genus Incrustatus (Anthozoa: Octocorallia: Clavulariidae) from Tierra del Fuego, Argentina, revealed by DNA barcoding. Helgoland Marine Research, 67(1): 137-147.
DOI:10.1007/s10152-012-0310-7 |
Nutting C C. 1912. Descriptions of the Alcyonaria collected by the U. S. Fisheries steamer Albatross, mainly in Japanese waters, during 1906. Proceedings of the United States National Museum, 43(1923): 1-104.
DOI:10.5479/si.00963801.43-1923.1 |
Rambaut A, Drummond A J. 2007. Tracer v1.4. Available at. http://beast.bio.ed.ac.uk/Tracer.
|
Ronquist F, Huelsenbeck J P. 2003. MrBayes 3:Bayesian phylogenetic inference under mixed models. Bioinformatics, 19(12): 1 572-1 574.
DOI:10.1093/bioinformatics/btg180 |
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30(12): 2 725-2 729.
DOI:10.1093/molbev/mst197 |
Tang Z C, 2008. Subphylum Anthozoa Ehrenberg, 1834. In: Liu J Y ed. Checklist of marine biota of China seas. Science Press, Beijing. p. 332-363.
|
Thomson J A, Henderson W D. 1906. An Account of the Alcyonarians Collected by the Royal Indian Marine Survey Ship Investigator in the Indian Ocean. Calcutta. 132p.
|
Verrill A E. 1865. Classification of polyps (extract condensed from Synopsis of the Polyps and Corals of the North Pacific Exploring Expedition under Commodore C. Ringgold and Captain John Rodgers, U.S.N.). . Communications of the Essex Institute, 4: 145-152.
|
Verrill A E. 1868. Notes on Radiata in the museum of Yale college, with descriptions of new genera and species. 4 notice of the corals and echinoderms collected by Prof. C.F. hartt, at the Abrolhos reefs, Province of Bahia, Brazil, 1867. Transactions of the Connecticut Acadademy of Arts and Sciences, 1(2): 351-613.
|
Williams G C, Alderslade P. 2011. Three new species of pennatulacean octocorals with the ability to attach to rocky substrata (Cnidaria: Anthozoa: Pennatulacea). Zootaxa, 3001(1): 33-48.
DOI:10.11646/zootaxa.3001.1.2 |
Williams G C. 1993. Biotic diversity, biogeography, and phylogeny of pennatulacean octocorals associated with coral reefs in the Indo-Pacific. In: Proceedings of the Seventh International Coral Reef Symposium. University of Guam Press, Mangilao. p. 729-736.
|
Williams G C. 1995. Living genera of sea pens (Coelenterata: Octocorallia: Pennatulacea): illustrated key and synopses. Zoological Journal of the Linnean Society, 113(2): 93-140.
DOI:10.1111/j.1096-3642.1995.tb00929.x |
Williams G C. 1997. Preliminary assessment of the phylogeny of Pennatulacea (Anthozoa: Octocorallia), with a reevaluation of Ediacaran frond-like fossils, and a synopsis of the history of evolutionary thought regarding the sea pens. In: Proceedings of the Sixth International Conference on Coelenterate Biology. Nationaal Natuurhistorisch Museum, Leiden. p. 495-509.
|
Williams G C. 2011. The global diversity of sea pens (Cnidaria: Octocorallia: Pennatulacea). PLoS One, 6(7): e22747.
DOI:10.1371/journal.pone.0022747 |
Williams G C. 2015. A new genus and species of pennatulacean octocoral from equatorial West Africa (Cnidaria, Anthozoa, Virgulariidae). Zookeys, 546: 39-50.
DOI:10.3897/zookeys.546.6344 |
Xu K D, Dong D, Gong L, et al. 2020. Photographic Atlas of Megafauna on Yap-Mariana-Caroline Seamounts in the Western Pacific Ocean. Science Press, Beijing. 239p.
(in Chinese)
|
 2021, Vol. 39
2021, Vol. 39



