Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHANG Yang, WANG Youquan, LIU Xiaoyu, DING Beichen, SUN Yi, CHANG Yaqing, DING Jun
- Metabolomics analysis for skin ulceration syndrome of Apostichopus japonicus based on UPLC/Q-TOF MS
- Journal of Oceanology and Limnology, 39(4): 1559-1569
- http://dx.doi.org/10.1007/s00343-020-0205-4
Article History
- Received May. 25, 2020
- accepted in principle Jul. 22, 2020
- accepted for publication Sep. 3, 2020
Sea cucumber (Apostichopus japonicus) is an important aquaculture species, with high nutritional and medicinal value. Since the 1980s, aquaculture of sea cucumber has rapidly developed, becoming one of the most important aquaculture species in China (Yu et al., 2014). In China, the annual production of sea cucumber was up to 1.74×105 t in 2018 (Zhang et al., 2019), but diseases caused by bacteria, virus, and protozoa bring enormous economic loss to aquaculture, which is one of the major factors limiting the productivity and development (Li et al., 2012). In addition, skin ulceration syndrome (SUS) is the most serious of all diseases due to its high infectivity and mortality of 90%–100% (Deng et al., 2008). Vibrio splendidus is a widely recognized pathogen responsible for SUS outbreaks (Liu et al., 2010). Therefore, V. splendidus was used in this study.
Currently, SUS in A. japonicus has been investigated from multiple aspects. For example, Zhang et al. (2013) studied SUS related miRNA targets using a transcription technique and Lv et al. (2019) analyzed SUS with proteomics. To fully understand the characteristics and pathogenesis of the disease, A. japonicus with SUS were analyzed using metabolomics in this study. Metabonomics is a qualitative and quantitative analysis of all metabolites in the whole organism, tissue, or single cell to find target differential metabolites under specific physiological cycles or conditions (Yan et al., 2019). There have been multiple studies using metabolomics. For example, Wikoff et al. (2009) analyzed the influence of intestinal flora on blood metabolites in mammals using metabolomics. Sabatine et al. (2005) used metabolomics to find labeled metabolites for myocardial ischemia disease. Metabolomics has been applied in the investigation of A. japonicus, including metabolic responses to stresses such as high temperature and hypoxia (Huo et al., 2019) and heat stress on the intestinal tract (Xu et al., 2017).
Recently, ultra-performance liquid chromatography (UPLC) and quadrupole-time of flight (Q-TOF) mass spectrometry (MS) has been widely applied in metabolomics studies due to its high sensitivity, good repeatability, high limit of detection, handling capacity, and chromatographic resolution. For example, Wang et al. (2008b) analyzed the constituents in the rat plasma after oral administration with Yin Chen Hao Tang (a classical traditional chinese medicine formula), Yin et al. (2006) investigated metabolomics in the intestinal fistula, Gu et al. (2019) determined the progestin residues in fish by UPLC/QTOF MS. In the present study, we investigated SUS in the sea cucumber A. japonicus, by assessing metabolites in the body wall using UPLC/Q-TOF MS and using the Assay Kit to detect the activities of superoxide dismutase (SOD), catalase (CAT), and level of malondialdehyde (MDA) in coelomic fluid. The results will help us to understand SUS, and provide reference data for investigating its characteristics and pathogenesis.
2 METHOD 2.1 Ethics statementThe sea cucumbers A. japonicus here are commercially cultured animals. All the works were conducted in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
2.2 AnimalAdult sea cucumber A. japonicus individuals (weight: 96.91±10.56 g) were collected from Dalian Xinyulong Aquaculture Company (39°20′N, 122°20′E), Liaoning, China in May 2019. Forty sea cucumbers were acclimatized in two tanks (200 cm× 50 cm×50 cm) with aerated, filtered water (16 ℃, salinity 30) for 7 days before use and were fed a commercial diet (Peng Anyuan Marine Food Co., Ltd., Yantai, China) once a day. Uneaten feed was removed daily during the acclimation and experimental periods. The acclimatized animals were then subjected to artificial infection.
2.3 Artificial infection and sample collectionVibrio splendidus used in infection experiments was obtained from the Key Laboratory of Mariculture & Stock Enhancement in Northern China Sea, Ministry of Agriculture and Rural Affairs, Dalian Ocean University. The bacteria were cultivated in 2216E liquid medium (Tryptone 5 g/L, yeast extract 1 g/L, C6H5Fe·5H2O 0.1 g/L, pH 7.6) with 150 r/min at 28 ℃ for 24 h and centrifuged at 5 000×g for 5 min to harvest the bacteria. For the challenge experiment, live bacteria were re-suspended in filtered seawater and adjusted to 1×1010 CFU/mL (The data obtained were statistically evaluated with Probit Analysis method (SPSS 20.0) and the 50% lethal concentration (LC50; 10 d; 0.2 mL) for sea cucumber Apostichopus japonicus exposed to the V. splendidus, was 4.35× 109 CFU/mL in our pretest.). After 7 days of acclimation, all animals were randomly divided into the normal group and the SUS group with 20 sea cucumbers in each group. And the normal group was injected with 0.2-mL 0.9% saline and the SUS group was injected with 0.2-mL 1×1010 CFU/mL of V. splendidus. 36 h after the injection, the animals shook their heads and their mouths swelled. Three days after the infection, most animals showed small patches of ulcer. A week after the infection, all the animals exhibited several deep and extensive white ulceration and displayed evisceration. We selected eight sea cucumbers with white skin ulceration and eight healthy sea cucumbers for further experiments. According to previous data (Jiang et al., 2009; Yang et al., 2016), white skin ulceration in sea cucumbers was considered the most important marker in identifying SUS and the coelomic fluid of sea cucumber is the main site of immune response. For each sampled individual, the coelomic fluid was collected immediately, split into five 2-mL tubes and one tube stored at -80 ℃ for osmolarity measuring. The remaining coelomic fluid was centrifuged at 800×g for 10 min at 4 ℃, the resulting supernatant was collected and used as coelomic fluid supernatant for detecting SOD, CAT activities and MDA content (Jiang et al., 2017). Some pieces of body wall (3 g in all) were separated and stored at -80 ℃ before metabolite extraction.
2.4 SOD and CAT activities and MDA contentThe coelomic fluid supernatant was taken at -80 ℃ and then dissolved at 4 ℃. After dissolving at 4 ℃, the activities of SOD, CAT, and MDA were detected using an Epoch microplate reader (BioTek, Winooski, VT, USA) with the SOD Assay Kit (A001-3-2, Jiancheng, Nanjing, China), CAT Assay Kit (A007-1- 1, Jiancheng, Nanjing, China), and MDA Assay Kit (A003-1-2, Jiancheng, Nanjing, China) (Wang et al., 2020; Zhou et al., 2020).
The SOD activity was assayed in water-soluble tetrazolium-1 (WST-1) method. One unit of SOD activity was defined as the amount of enzyme required for 1-mg tissue proteins in 1 mL of a reaction mixture SOD inhibition rates to 50% as monitored at 450 nm. CAT activity was admeasured by using ammonium molybdate colorimetric method, which was measured at 405 nm. One unit of CAT activity was defined as 1-mg tissue proteins consumed 1-μmol H2O2 per second. We used thiobarbituric acid (TBA) method to determine MDA content at 532 nm.
2.5 Osmolarity measurementsAfter the coelomic fluid thawed at 4 ℃, 50 μL of coelomic fluid was taken into a centrifuge tube. Osmomat 030 (Gonotec, Berlin, Germany) was used to detect the osmotic pressure of the sample. Each sample was measured three times and the average value was taken. One unit of osmotic pressure was defined as 1-mmol solute is dissolved in 1-kg solvent.
2.6 Sample preparation for metabolomic analysisSamples were taken at -80 ℃ and then 80 mg of every sample was weighed, homogenized in 200-μL water using a FastPrep-24 5G homogenizer (MP, USA). After vortexing, 800 μL of methanol/ acetonitrile (1∶1, v/v) was added to the samples. The samples were subjected twice to 30 min cryogenic sonication treatment followed by vortexing for 60 s. Then let it stand for 1 h at -20 ℃ and centrifuged at 14 000×g for 20 min at 4 ℃ in a 5430R ultracentrifuge (Eppendorf, Germany) to obtain the supernatant. The supernatant was freeze-dried and stored at -80 ℃ before further analysis.
2.7 UHPLC-Q-TOF MS spectrometry analysisThe metabolic profiling analysis of the tissues was conducted on an Agilent 1290 Infinity UPLC system (Agilent, USA) with an ACQUITY UPLC BEH Amide column (1.7 μm, 2.1 mm×100 mm) under constant flow rate of 0.3 mL/min. The column temperature was set to 25 ℃. The mobile phase consisted of aqueous ammonium acetate (25 mmol/L)/ ammonia (25 mmol/L) (A) and acetonitrile (B). The gradient elution was: 0–1 min, 95% B; 1–14 min, 95–65% B; 14–16 min, 65%–40% B; 16–18 min, 40% B; 18–18.1 min, 40%–95% B; and 18.1–23 min, 95% B. A Triple TOF 5600 mass spectrometer (AB SCIEX, USA) was used to detect the metabolites eluted from the column, and the MS was performed to analyze the supernatant in positive and negative ion mode. The electrospray ionization (ESI) source operation parameters were: ion source gas1 (Gas1): 60, ion source gas2 (Gas2): 60, curtain gas (CUR): 30, source temperature: 600 ℃, ion spray voltage floating (ISVF) 60, curtain gas (CUR): 30, source temperature: 600. The electrospray: 1 000 Da, product ion scan m/z range: 25–1 000 Da, TOF MS scan accumulation time 0.20 s/spectra, product ion scan accumulation time 0.05 s/spectra. MS/MS data were obtained from an information-dependent acquisition (IDA), operating in a high-sensitivity mode. Declustering potential (DP): ±60 V (positive and negative ion mode), collision energy: 35±15 eV, and exclude isotopes within 4 Da, candidate ions to monitor per cycle: 6 was used in informationdependent acquisition.
All MS data were conducted with three analytical replications (Zhao et al., 2017).
2.8 Data processingThe initial MS data were converted into extensible markup language (mzXML) format using the ProteoWizard, and then the xml cryptographic message syntax (XCMS) program was used for peak alignment, retention time correction, and peak area extraction. The method of MS/MS data matching and accurate mass matching (< 25×10-6) to retrieve a selfbuilt database from the laboratory was used to identify and authenticate the metabolite structure.
The analysis methods included the principal component analysis (PCA) and orthogonal partial least-squares discriminant analysis (OPLS-DA). All data were subject to preprocessing by Pareto scaling before analyses (Yan et al., 2019). The online databases Kyoto Encyclopedia of Genes and Genomes (KEGG) (http://www.genome.jp/kegg/pathway.html) (Kanehisa et al., 2015) were used to identify possible metabolic pathways.
2.9 Statistical analysisFor the metabolomics analysis, Student's t-test was conducted to select the differential metabolites based on the variable importance in the projection (VIP) values > 1 and the P-value < 0.1 (Hao et al., 2018). For the SOD, CAT activities and MDA content analyses, all samples were analyzed and data were averaged, and the results are expressed as the mean±SD. Significant differences (P < 0.05) and highly significant differences (P < 0.01) for each variable were detected using Student's t-test. Statistical analysis was performed using Excel (Microsoft, USA) and SPSS 20.0 (IBM, USA).
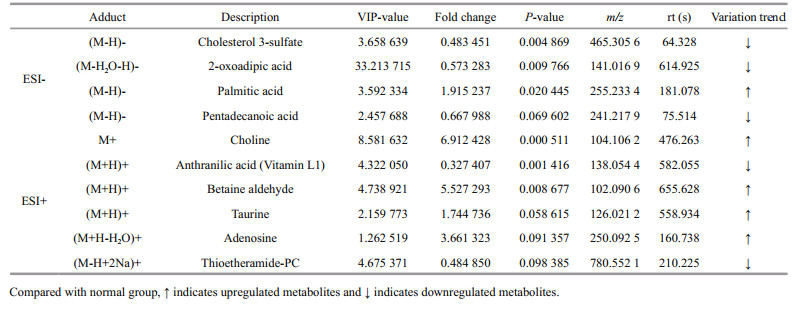
3 RESULT 3.1 Activities of SOD and CAT, and level of MDAActivities of CAT and SOD, MDA content in coelomic fluid were detected in the normal and SUS group. As shown in Fig. 1, the activities of CAT and SOD were reduced (P < 0.05) compared with the normal group but the level of MDA was increased (P < 0.01).
|
| Fig.1 Influence of SUS on SOD activity (a), CAT activity (b) and MDA content (c) in coelomic fluid of A. japonicas Compared with normal group, * indicates significant differences (P < 0.05), ** indicates highly significant differences (P < 0.01). |
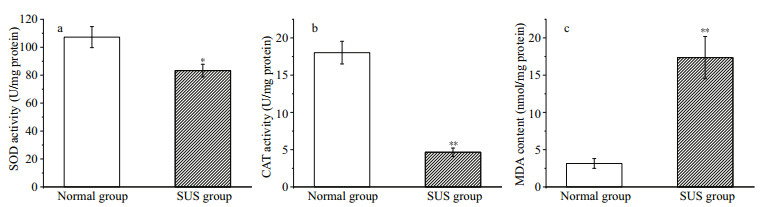
There were significant differences in the osmolarity values of coelomic fluid collected from two groups of the study sea cucumbers (Fig. 2). Differences were also detected following Student's t-test (P < 0.01).
|
| Fig.2 The osmolarity values (Os mol/kg) for two groups of the sea cucumber Compared with normal group, ** indicates highly significant differences (P < 0.01). |
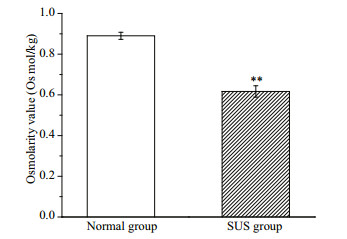
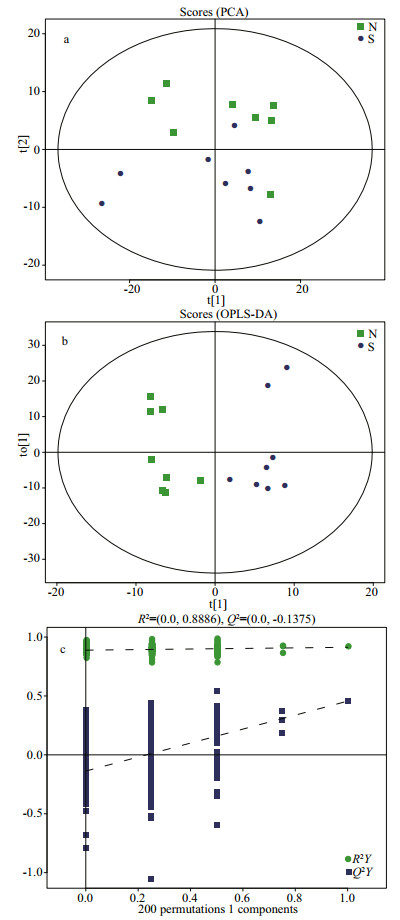
The total ion chromatography (TIC) results of the normal and SUS groups are presented in Fig. 3. In total, 579 metabolites were identified, and some differences in terms of peaks heights were observed between two groups. The differences in the metabolites of the body wall can be discriminated by the pattern recognition method, such as PCA and OPLS-DA models. As shown in Fig. 4b, the OPLS-DA scores plot for all groups is excellently clustered and grouped without any overlap. R2X, R2Y, and Q2 are 0.494 cum, 0.991 cum, and 0.604 cum. In permutation testing (Fig. 4c), the values of R2 and Q2 were 0.888 6 and -0.137 5, indicating good repeatability and predictability of the model.
|
| Fig.3 Total ion current (TIC) chromatograms of the samples a. normal group; b. skin ulceration syndrome group. |
|
| Fig.4 PCA (a) and OPLS-DA (b) score plots of normal group (square, green), skin ulceration syndrome group (round, blue), permutation test of OPLS-DA model (c) |
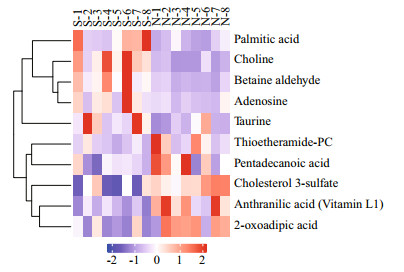
A total of 10 differential metabolites were finally detected (VIP > 1 and P < 0.1; Fig. 5 & Table 1), the levels of metabolites, including adenosine, choline, betaine aldehyde, palmitic acid, and taurine were upregulated, compared with the normal group, whereas, 2-oxoadipic acid, anthranilic acid (Vitamin L1), thioetheramide-PC, cholesterol 3-sulfate, and pentadecanoic acid were down-regulated.
|
| Fig.5 Hierarchical clustering analysis for SDMs The relative metabolite level is depicted according to color scale. Red indicates upregulation, and blue indicates downregulation. The S represents SUS group, and the N represents normal group. |
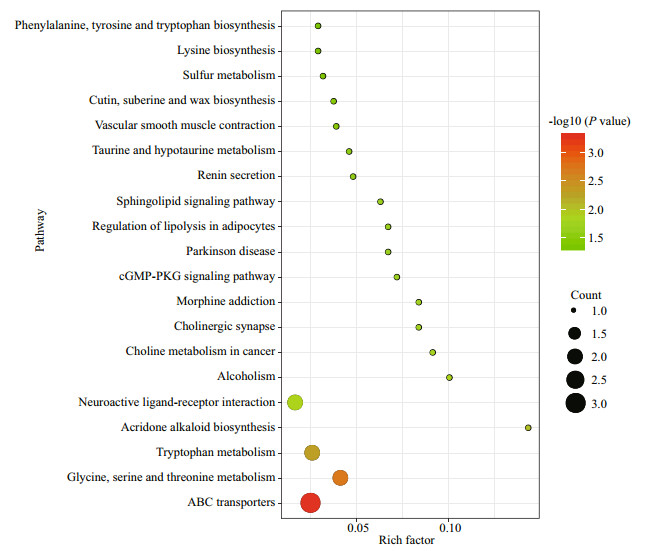
The screened differential metabolites were input into the KEGG database, and KEGG pathway enrichment analysis was performed via Fisher's precise test. All enrichment pathways are illustrated in Fig. 6. Moreover, the enrichment of KEGG pathways was mainly related to amino acid metabolism (glycine, serine, and threonine metabolism, Tryptophan metabolism and Taurine and hypotaurine metabolism) between two groups.
|
| Fig.6 KEGG pathway enrichment analysis of differential metabolites between two groups |
It has been reported that sea cucumber recognizes, inhibits, kills, and eliminates pathogens by immune factors in the organism (mainly enzymes) due to the lack of a specific immune system (Wang et al., 2008a).
SOD and CAT are important antioxidant enzymes in invertebrates (Roch, 1999). In this experiment, the activities of SOD and CAT in the SUS group were significantly reduced compared to the normal (P < 0.05). Lv et al. (2019) analyzed A. japonicus with SUS using proteomics and also found that levels of the two enzymes were downregulated. It indicated that the effects of SOD and CAT in sea cucumber with SUS were blocked, the capability of scavenging free radicals declined, and the defense response was reduced, which might be detrimental to the organism.
MDA can damage the function of tissues and cells, indicating lipid peroxidation and impaired cell function (Cossu et al., 2000). In this study, the MDA level was significantly increased in the SUS group compared with the normal group (P < 0.01), which was the same as the change of MDA level when A. japonicus is under hypoxic stress (Huo et al., 2018). The results demonstrated that lipid peroxidation in A. japonicus with SUS increases with severe cell injury.
Overall, the activities of CAT and SOD were reduced (P < 0.05) compared with the normal group but the level of MDA was increased (P < 0.01). These results indicated that SUS changed the non-specific immunity of the organism.
4.2 Analysis of differential metabolitesAdenosine can prevent or reduce the increased permeability of the endothelial monolayer, and further alleviate the damage to function by oxidation (Richard et al., 1998). Adenosine has been reported to alleviate injury in multiple types of animal (Lasley et al., 1990; Baldissera et al., 2018). In this study, sea cucumbers in the SUS group showed some white speaks of skin ulceration. It can be inferred that the adenosine level was upregulated (VIP > 1 and P < 0.1) in the SUS group in order to alleviate cell injury caused by SUS.
Glycine betaine is an organic penetrant synthesized by a two-step reaction from choline: choline → betaine aldehyde + nicotinamide adenine dinucleotide (NAD+) + → glycine betaine + dihydronicotinamide adenine dinucleotide (NADH), which plays a role in regulating osmotic equilibrium in marine invertebrates (Perrino and Pierce, 2000). Taurine is also an organic penetrant. In previous study, V. splendidus has been reported to cause osmoregulatory change in Ruditapes philippinesis (Liu et al., 2013). As showed in Fig. 2, the osmolarity values of coelomic fluid collected from two groups were significantly different (P < 0.01). Therefore we propose that the levels of choline, betaine aldehyde, and taurine in A. japonicus were upregulated (VIP > 1 and P < 0.1) in the SUS group compared with the normal group, causing osmoregulatory change in A. japonicus.
Palmitic acid has been found to cause oxidative stress in cells via damage to the normal function of mitochondrial oxidative respiratory chain (Win et al., 2015). It can be also used as an efficient proinflammatory factor to induce an inflammationrelated cascade reaction (Pillon et al., 2015; Ma et al., 2018). It was reported that there was a change in the proinflammatory response and expression of immunity-related genes in SUS (Zhang et al., 2013). Thus, upregulation of palmitic acid in the SUS group might lead to an inflammatory response and oxidative stress, damaging the organism and influencing immunocompetence. The 2-oxoadipic acid is a metabolite of tryptophan decomposition (Shibata et al., 2011). Anthranilic acid (Vitamin L1) plays a very important role in the biosynthesis of tryptophan (Wiklund and Bergman, 2006). Tryptophan, as a functional essential amino acid, plays important roles in enhancing anti-oxidative and immune functions in animals (Li et al., 2016). In this study, the levels of 2-oxoadipic acid and anthranilic acid (Vitamin L1) in the SUS group were downregulated (VIP > 1 and P < 0.1) compared to those in the normal group, which might influence anti-oxidative and immune functions by influencing tryptophan metabolism. Moreover, the changes of SOD, CAT activities and MDA content supports these speculations, compared with normal group, the immunocompetence of SUS group has changed. Based on available data, pentadecanoic acid can influence energy metabolism (Pfeuffer and Jaudszus, 2016). The change of energy metabolism in sea cucumber with SUS was proven (Shao et al., 2013; Zhao et al., 2017). We infer the level of pentadecanoic acid downregulated is one cause for the change of energy metabolism.
Low concentrations thioetheramide-PC can activate phospholipase A2 (sPLA2) activity (Xie et al., 2005). Recent studies suggest, sPLA2 promote several inflammatory diseases (Mallat et al., 2010) and the increase of sPLA2 was observed in aquatic organisms with Aeromonas hydrophila (Hu et al., 2005). The down of thioetheramide-PC may promote SUS through sPLA2 activity. The cholesterol-3- sulfate is a natural steroid and the steroid from marine echinoderms has antiviral effect (Roccatagliata et al., 1996). It can be inferred that the cholesterol-3-sulfate may also has antiviral effect in sea cucumber and the down of cholesterol-3-sulfate will promote SUS.
4.3 Metabolic pathway analysisIn this work, we used KEGG annotation for performing enrichment analyses to investigate the functional distribution of identified metabolites and the significant enrichment results of KEGG pathway were related mainly to energy metabolism, immunity, and osmoregulation between two groups.
ABC transporters and neuroactive ligand-receptor interaction were the pathways of environmental information processing. ATP-binding cassette (ABC) transporters are a large group of membrane proteins that couple transport of a substrate against a chemical gradient and energized directly by the hydrolysis of ATP (Borths et al., 2002; Kathawala et al., 2015). ABC transporters play an important role in protective function by reducing the accumulation of toxins in the body (Ejendal and Hrycyna, 2002). Studies shown that aquatic animals can efflux harmful substances out of body in time and reduce the body damage by ABC transporters (Zhou et al., 2009; Chang et al., 2012). Therefore, we infer that sea cucumber may also use ABC transporters to efflux V. splendidus out of body, which reduce the body damage. Neuroactive ligandreceptor interaction pathway is a signal transduction pathway composed of all ligands and receptors in cell membrane (Lauss et al., 2007). It was reported that Eriocheir sinensis with Spiroplasma eriocheiris infection characterized by trembling was associated with the neuroactive ligand-receptor interaction pathway (Wang et al., 2017). Head-shaking is one of the symptoms of sea cucumber with SUS (Zhang et al., 2006). Based on these reports, we speculate that the neuroactive ligand-receptor interaction pathway may be responsible for the sea cucumber sharks head during SUS. However, the detailed regulation role of this pathway needs to be elucidated in the future.
Glycine, serine and threonine metabolism and tryptophan metabolism were the pathways of amino acid metabolism. In marine invertebrates, amino acid is regarded as a main participant regulating energy metabolism and osmotic pressure (Viant et al., 2003). Generally, high concentrations of amino acids can be used to regulate osmotic pressure as a penetrant. When the invertebrates are exposed to various types of stress, the energy requirement is increased, which can be met by the energy generated by the oxidation of amino acids (Lv et al., 2019). This indicates that energy metabolism and regulatory capability of osmotic pressure in sea cucumber with SUS may change, which is consistent with current studies (Shao et al., 2013; Zhao et al., 2017).
5 CONCLUSIONThe activities of SOD and CAT and the level of MDA in coelomic fluid in the SUS group and normal group were detected. Results indicate that the level of MDA in the SUS group was significantly increased (P < 0.01), but the activities of SOD and CAT were significantly reduced (P < 0.05). In addition, the nonspecific immunity was changed in the sea cucumber with SUS. Levels of metabolites, such as adenosine, choline, betaine aldehyde, palmitic acid, and taurine, were upregulated. However, 2-oxoadipic acid, anthranilic acid (vitamin L1), thioetheramide-PC, cholesterol-3-sulfate, and pentadecanoic acid were downregulated (VIP > 1 and P < 0.1). These different metabolites are related mainly to alleviating injury, regulating osmotic pressure, and influencing immunocompetence. KEGG pathway enrichment analysis showed most enrichment of KEGG pathways are related t energy metabolism, immunity, and osmoregulation. There were significant differences in the osmolarity values of coelomic fluid between the two groups. Therefore, this study indicates that energy metabolism, immunologic function and osmotic pressure regulation change in A. japonicus with SUS. Results of this study could help understand the characteristics of SUS, and provide a reference for further investigations.
6 DATA AVAILABILITY STATEMENTAll data generated or analyzed during this study are included in this article.
7 CONFLICT OF INTERESTThe authors declare that there is no conflict of interests regarding the publication of this article.
Baldissera M D, Souza C F, Doleski P H, Monteiro S G, da Silva A S, Baldisserotto B. 2018. Serum adenosine deaminase and xanthine oxidase activities in silver catfish naturally infected with Ichthyophthirius multifiliis: the influence of these enzymes on inflammatory and oxidative status. Journal of Fish Diseases, 41(2): 263-268.
DOI:10.1111/jfd.12709 |
Borths E L, Locher K P, Lee A T, Rees D C. 2002. The structure of Escherichia coli BtuF and binding to its cognate ATP binding cassette transporter. Proceedings of the National Academy of Sciences of the United States of America, 99(26): 16 642-16 647.
DOI:10.1073/pnas.262659699 |
Chang Z Q, Li J, Liu P, Kuo M M C, He Y Y, Chen P, Li J T. 2012. cDNA cloning and expression profile analysis of an ATP-binding cassette transporter in the hepatopancreas and intestine of shrimp Fenneropenaeus chinensis. Aquaculture, 356-357: 250-255.
DOI:10.1016/j.aquaculture.2012.05.009 |
Cossu C, Doyotte A, Babut M, Exinger A, Vasseur P. 2000. Antioxidant biomarkers in freshwater bivalves, Unio tumidus, in response to different contamination profiles of aquatic sediments. Ecotoxicology and Environmental Safety, 45(2): 106-121.
DOI:10.1006/eesa.1999.1842 |
Deng H, He C B, Zhou Z C, Liu C, Tan K F, Wang N B, Jiang B, Gao X G, Liu W D. 2008. Isolation and pathogenicity of pathogens from skin ulceration disease and viscera ejection syndrome of the sea cucumber Apostichopus japonicus. Aquaculture, 287(1-2): 18-27.
DOI:10.1016/j.aquaculture.2008.10.015 |
Ejendal K F, Hrycyna C A. 2002. Multidrug resistance and cancer: the role of the human ABC transporter ABCG2. Current Protein and Peptide Science, 3(5): 503-511.
DOI:10.2174/1389203023380521 |
Gu C X, Cheng Y L, Zhen X, Chen X X, Zhou K W. 2019. Determination of progestin residues in fish by UPLC-Q-TOF/MS coupled with QuEChERS. Journal of Analytical Methods in Chemistry, 2019: 6426958.
DOI:10.1155/2019/6426958 |
Hao R J, Wang Z M, Yang C Y, Deng Y W, Zheng Z, Wang Q H, Du X D. 2018. Metabolomic responses of juvenile pearl oyster Pinctada maxima to different growth performances. Aquaculture, 491: 258-265.
DOI:10.1016/j.aquaculture.2018.03.050 |
Hu C H, Xia M S, Xiong L, Xu X R. 2005. Effects of Cu bearing montmorillonite on Aeromonas hydrophila adhesion to epithelial cells of Nile tilapia. Journal of Fisheries of China, 29(5): 619-623.
(in Chinese with English abstract) DOI:10.3321/j.issn:1000-0615.2005.05.006 |
Huo D, Sun L, Ru X S, Zhang L B, Lin C G, Liu S L, Xin X K, Yang H S. 2018. Impact of hypoxia stress on the physiological responses of sea cucumber Apostichopus japonicus: respiration, digestion, immunity and oxidative damage. PeerJ, 6: e4651.
DOI:10.7717/peerj.4651 |
Huo D, Sun L, Zhang L B, Ru X S, Liu S L, Yang H S. 2019. Metabolome responses of the sea cucumber Apostichopus japonicus to multiple environmental stresses: heat and hypoxia. Marine Pollution Bulletin, 138: 407-420.
DOI:10.1016/j.marpolbul.2018.11.063 |
Jiang J W, Zhou Z C, Dong Y, Jiang B, Chen Z, Gao S, Guan X Y, Han L. 2017. Seasonal variations of immune parameters in the coelomic fluid of sea cucumber Apostichopus japonicus cultured in pond. Aquaculture Research, 48(4): 1 677-1 687.
DOI:10.1111/are.13005 |
Jiang X L, Du Y S, Wang P, Liu R Z, Yang X S, Lv Q. 2009. Effects of alginate-derived oligosaccharide on the activities of immunoenzymes in the coelomic fluid and body wall of sea cucumber (Apostichopus japonicus). Periodical of Ocean University of China, 39(6): 1 188-1 192.
(in Chinese with English abstract) DOI:10.3969/j.issn.1672-5174.2009.06.006 |
Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. 2015. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Research, 44(D1): D457-D462.
DOI:10.1093/nar/gkv1070 |
Kathawala R J, Gupta P, Ashby C R Jr, Chen Z S. 2015. The modulation of ABC transporter-mediated multidrug resistance in cancer: a review of the past decade. Drug Resistance Updates, 18: 1-17.
DOI:10.1016/j.drup.2014.11.002 |
Lasley R D, Rhee J W, Van Wylen D G L, Mentzer R M Jr. 1990. Adenosine A1 receptor mediated protection of the globally ischemic isolated rat heart. Journal of Molecular and Cellular Cardiology, 22(1): 39-47.
DOI:10.1016/0022-2828(90)90970-D |
Lauss M, Kriegner A, Vierlinger K, Noehammer C. 2007. Characterization of the drugged human genome. Pharmacogenomics, 8: 1 063-1 073.
DOI:10.2217/14622416.8.8.1063 |
Li C H, Feng W D, Qiu L H, Xia C G, Su X R, Jin C H, Zhou T T, Zeng Y, Li T W. 2012. Characterization of skin ulceration syndrome associated microRNAs in sea cucumber Apostichopus japonicus by deep sequencing. Fish and Shellfish Immunology, 33(2): 436-441.
DOI:10.1016/j.fsi.2012.04.013 |
Li H W, Zhu Q, Wu L Y, Yin Y L, Kong X F. 2016. Physiological function and dietary application of tryptophan in livestock and poultry. Chinese Journal of Animal Nutrition, 28(3): 659-664.
(in Chinese with English abstract) DOI:10.3969/j.issn.1006-267x.2016.03.004 |
Liu H, Zheng F, Sun X, Hong X, Dong S, Wang B, Tang X, Wang Y. 2010. Identification of the pathogens associated with skin ulceration and peristome tumescence in cultured sea cucumbers Apostichopus japonicus (Selenka). Journal of Invertebrate Pathology, 105(3): 236-242.
DOI:10.1016/j.jip.2010.05.016 |
Liu X L, Ji C L, Zhao J M, Wu H F. 2013. Differential metabolic responses of clam Ruditapes philippinarum to Vibrio anguillarum and Vibrio splendidus challenges. Fish and Shellfish Immunology, 35(6): 2 001-2 007.
DOI:10.1016/j.fsi.2013.09.014 |
Lv Z M, Guo M, Li C H, Shao Y N, Zhao X L, Zhang W W. 2019. Divergent proteomics response of Apostichopus japonicus suffering from skin ulceration syndrome and pathogen infection. Comparative Biochemistry and Physiology — Part D: Genomics and Proteomics, 30: 196-205.
DOI:10.1016/jxbd.2019.03.003 |
Ma X Z, Pang Z D, Wang J H, Song Z, Zhao L M, Du X J, Deng X L. 2018. The role and mechanism of KCa3.1 channels in human monocyte migration induced by palmitic acid. Experimental Cell Research, 369(2): 208-217.
DOI:10.1016/j.yexcr.2018.05.020 |
Mallat Z, Lambeau G, Tedgui A. 2010. Lipoprotein-associated and secreted phospholipases A2 in cardiovascular disease. Circulation, 122(21): 2 183-2 200.
DOI:10.1161/CIRCULATIONAHA.110.936393 |
Perrino L A, Pierce S K. 2000. Betaine aldehyde dehydrogenase kinetics partially account for oyster population differences in glycine betaine synthesis. The Journal of Experimental Zoology, 286(3): 238-249.
DOI:10.1002/(SICI)1097-010X(20000215)286:3<238::AID-JEZ3>3.0.CO;2-E |
Pfeuffer M, Jaudszus A. 2016. Pentadecanoic and heptadecanoic acids: multifaceted odd-chain fatty acids. Advances in Nutrition, 7(4): 730-734.
DOI:10.3945/an.115.011387 |
Pillon N J, Azizi P M, Li Y E, Liu J, Wang C, Chan K L, Hopperton K E, Bazinet R P, Heit B, Bilan P J, Lee W L, Klip A. 2015. Palmitate-induced inflammatory pathways in human adipose microvascular endothelial cells promote monocyte adhesion and impair insulin transcytosis. American Journal of Physiology-Endocrinology and Metabolism, 309(1): E35-E44.
DOI:10.1152/ajpendo.00611.2014 |
Richard L F, Dahms T E, Webster R O. 1998. Adenosine prevents permeability increase in oxidant-injured endothelial monolayers. American Journal of Physiology—Heart and Circulatory Physiology, 274(1): H35-H42.
DOI:10.1152/ajpheart.1998.274.1.H35 |
Roccatagliata A J, Maier M S, Seldes A M, Pujol C A, Damonte E B. 1996. Antiviral sulfated steroids from the ophiuroid Ophioplocus januarii. Journal of Natural Products, 59(9): 887-889.
DOI:10.1021/np960171a |
Roch P. 1999. Defense mechanisms and disease prevention in farmed marine invertebrates. Aquaculture, 172(1-2): 125-145.
DOI:10.1016/S0044-8486(98)00439-6 |
Sabatine M S, Liu E, Morrow D A, Heller E, McCarroll R, Wiegand R, Berriz G F, Roth F P, Gerszten R E. 2005. Metabolomic identification of novel biomarkers of myocardial ischemia. Circulation, 112(25): 3 868-3 875.
DOI:10.1161/CIRCULATIONAHA.105.569137 |
Shao Y, Li C H, Ou C R, Zhang P, Lu Y L, Su X R, Li Y, Li T W. 2013. Divergent metabolic responses of Apostichopus japonicus suffered from skin ulceration syndrome and pathogen challenge. Journal of Agricultural and Food Chemistry, 61(45): 1 0766-1 0771.
DOI:10.1021/jf4038776 |
Shibata K, Yasui M, Sano M, Fukuwatari T. 2011. Fluorometric determination of 2-oxoadipic acid, a common metabolite of tryptophan and lysine, by high-performance liquid chromatography with pre-chemical derivatization. Bioscience, Biotechnology, and Biochemistry, 75(1): 185-187.
DOI:10.1271/bbb.100723 |
Viant M R, Rosenblum E S, Tjeerdema R S. 2003. NMR-based metabolomics: a powerful approach for characterizing the effects of environmental stressors on organism health. Environmental Science & Technology, 37(21): 4 982-4 989.
DOI:10.1021/es034281x |
Wang F Y, Yang H S, Gao F, Liu G B. 2008a. Effects of acute temperature or salinity stress on the immune response in sea cucumber, Apostichopus japonicus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 151(4): 491-498.
DOI:10.1016/j.cbpa.2008.06.024 |
Wang X J, Sun W J, Sun H, Lv H T, Wu Z M, Wang P, Liu L, Cao H X. 2008b. Analysis of the constituents in the rat plasma after oral administration of Yin Chen Hao Tang by UPLC/Q-TOF-MS/MS. Journal of Pharmaceutical and Biomedical Analysis, 46(3): 477-490.
DOI:10.1016/j.jpba.2007.11.014 |
Wang Y F, Yang J, Hu Y X, Zhang H B, Ding J, Wang L, Qiu X M. 2020. Effects of Salinities on Immune-related Indicators of Sea Cucumber (Apostichopus japonicus). Journal of Guangdong Ocean University, 40(3): 22-29.
(in Chinese with English abstract) DOI:10.3969/j.issn.1673-9159.2020.03.004 |
Wang Y H, Xiu Y J, Bi K R, Ou J T, Gu W, Wang W, Meng Q G. 2017. Integrated analysis of MRNA-seq in the haemocytes of Eriocheir sinensis in response to Spiroplasma eriocheiris infection. Fish & Shellfish Immunology, 68: 289-298.
DOI:10.1016/j.fsi.2017.07.036 |
Wiklund P, Bergman J. 2006. The chemistry of anthranilic acid. Current Organic Synthesis, 3(3): 379-402.
DOI:10.2174/157017906777934926 |
Wikoff W R, Anfora A T, Liu J, Schultz P G, Lesley S A, Peters E C, Siuzdak G. 2009. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proceedings of the National Academy of Sciences of the United States of America, 106(10): 3 698-3 703.
DOI:10.1073/pnas.0812874106 |
Win S, Than T A, Le B H A, Garcia-Ruiz C, Fernandez-Checa J C, Kaplowitz N. 2015. Sab (Sh3bp5) dependence of jnk mediated inhibition of mitochondrial respiration in palmitic acid induced hepatocyte lipotoxicity. Journal of Hepatology, 62(6): 1 367-1 374.
DOI:10.1016/j.jhep.2015.01.032 |
Xie Y L, Liu L H, Huang X C, Guo Y W, Lou L G. 2005. Scalaradial inhibition of epidermal growth factor receptor-mediated Akt phosphorylation is independent of secretory phospholipase A2. The Journal of Pharmacology and Experimental Therapeutics, 314(3): 1 210-1 217.
DOI:10.1124/jpet.105.086520 |
Xu D X, Zhou S, Yang H S. 2017. Carbohydrate and amino acids metabolic response to heat stress in the intestine of the sea cucumber Apostichopus japonicus. Aquaculture Research, 48(12): 5 883-5 891.
DOI:10.1111/are.13411 |
Yan N, Du Y M, Liu X M, Chu M J, Shi J, Zhang H B, Liu Y H, Zhang Z F. 2019. A comparative UHPLC-QqQ-MS-based metabolomics approach for evaluating Chinese and North American wild rice. Food Chemistry, 275: 618-627.
DOI:10.1016/j.foodchem.2018.09.153 |
Yang A F, Zhou Z C, Pan Y J, Jiang J W, Dong Y, Guan X Y, Sun H J, Gao S, Chen Z. 2016. RNA sequencing analysis to capture the transcriptome landscape during skin ulceration syndrome progression in sea cucumber Apostichopus japonicus. BMC Genomics, 17: 459.
DOI:10.1186/s12864-016-2810-3 |
Yin P Y, Zhao X J, Li Q R, Wang J S, Li J S, Xu G W. 2006. Metabonomics study of intestinal fistulas based on ultraperformance liquid chromatography coupled with Q-TOF mass spectrometry (UPLC/Q-TOF MS). Journal of Proteome Research, 5(9): 2 135-2 143.
DOI:10.1021/pr060256p |
Yu Z H, Zhou Y, Yang H S, Hu C Q. 2014. Bottom culture of the sea cucumber Apostichopus japonicus Selenka (Echinodermata: Holothuroidea) in a fish farm, southern China. Aquaculture Research, 45(9): 1 434-1 441.
DOI:10.1111/are.12089 |
Zhang C Y, Wang Y G, Rong X J. 2006. Isolation and identification of causative pathogen for skin ulcerative syndrome in Apostichopus japonicus. Journal of Fisheries of China, 30(1): 118-123.
(in Chinese with English abstract) DOI:10.3321/j.issn:1000-0615.2006.01.019 |
Zhang P J, Li C H, Zhu L, Su X R, Li Y, Jin C H, Li T W. 2013. De novo assembly of the sea cucumber Apostichopus japonicus hemocytes transcriptome to identify miRNA targets associated with skin ulceration syndrome. PLoS One, 8(9): e73506.
DOI:10.1371/journal.pone.0073506 |
Zhang X L, Cui L F, Li S M. 2019. China Fishery Statistical Yearbook (2019). China Agriculture Press, Beijing, China. p. 23. (in Chinese)
|
Zhao G H, Hou X L, Li X Y, Qu M, Tong C Q, Li W. 2017. Metabolomics analysis of alloxan-induced diabetes in mice using UPLC-Q-TOF-MS after Crassostrea gigas polysaccharide treatment. International Journal of Biological Macromolecules, 108: 550-557.
DOI:10.1016/j.ijbiomac.2017.12.057 |
Zhou J, He W Y, Wang W N, Yang C W, Wang L, Xin Y, Wu J, Cai D X, Liu Y, Wang A L. 2009. Molecular cloning and characterization of an ATP-binding cassette (ABC) transmembrane transporter from the white shrimp Litopenaeus vannamei. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 150(4): 450-458.
DOI:10.1016/j.cbpc.2009.06.012 |
Zhou L, Li H F, Qin J G, Wang X D, Chen L Q, Xu C, Li E C. 2020. Dietary prebiotic inulin benefits on growth performance, antioxidant capacity, immune response and intestinal microbiota in Pacific white shrimp (Litopenaeus vannamei) at low salinity. Aquaculture, 518: 734847.
DOI:10.1016/j.aquaculture.2019.734847 |
 2021, Vol. 39
2021, Vol. 39