Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CAO Qianjin, LIU Beibei, HU Feiyang
- Effects of hydrological connection and human disturbance on genetic variation of submerged Vallisneria natans populations in four lakes in China
- Journal of Oceanology and Limnology, 39(4): 1403-1416
- http://dx.doi.org/10.1007/s00343-020-0277-1
Article History
- Received Jul. 17, 2020
- accepted in principle Sep. 23, 2020
- accepted for publication Nov. 2, 2020
With the increase in the need for flood prevention and lake resource used by humans, the construction of floodgates and sluices has changed the hydrological connection between rivers and lakes, and between adjacent lakes (Wang and Dou, 1998; Lacoul and Freedman, 2006; Chen et al., 2009). Many lakes, historically connected with rivers, have become disconnected and some, isolated in recent decades (Wang and Dou, 1998). The hydrologic variations of the lakes, caused by sluices, are markedly different from the original natural ones (Lacoul and Freedman, 2006). In fact, floodgates and sluices reduce peak flow and the magnitude of water level fluctuation (Nilsson et al., 2010), and prolong water retention time, thus accumulating more pollutants in the lake water. Additionally, artificial control of water level via sluices facilitates intense fishing and favors lake enclosures for cultivation of certain crops, resulting in anthropogenic mechanical disturbance and water quality decline to different extents (Qin, 2002). Therefore, human activities greatly change the aquatic habitat conditions of submerged plants (Chambers et al., 2007; Rejmánková, 2011). Environmental characteristics of aquatic habitats influence the occurrence of submerged species and their life-history traits (recruitment, growth, and reproduction) (Bornette and Puijalon, 2010). Moreover, sluices affect the hydrochory of submerged plants by acting as physical barriers to dispersal (Nilsson et al., 2010). Hence, anthropogenic changes in aquatic environmental conditions may affect the genetic variation of submerged species in lakes.
With the reputation of being "the province with thousands of lakes", the Hubei Province, in central China, does indeed have a large number of shallow lakes lying in a subtropical monsoon climate (Committee for Lake Records of Hubei Province, 2014). Of the lakes in the middle reach of the Changjiang (Yangtze) River (the longest river in China), the Futou Lake and the Xiliang Lake lie close together, and both belong to the water system of the Jinshui River (Fig. 1, Table 1). The two lakes were components of one large ancient lake before the 16th century and, historically, directly connected to each other by a narrow waterway until the 1950s (Committee for Lake Records of Hubei Province, 2014). Their water mingles with the Jinshui River before reaching the Changjiang River. Similarly, Liangzi Lake and Baoan Lake are also closely related, historically, to a large ancient lake and remain directly interconnected, belonging to the same water system (Liangzi Lake Water System). Their water mingles with the Changgang River, which drains into the Changjiang River.
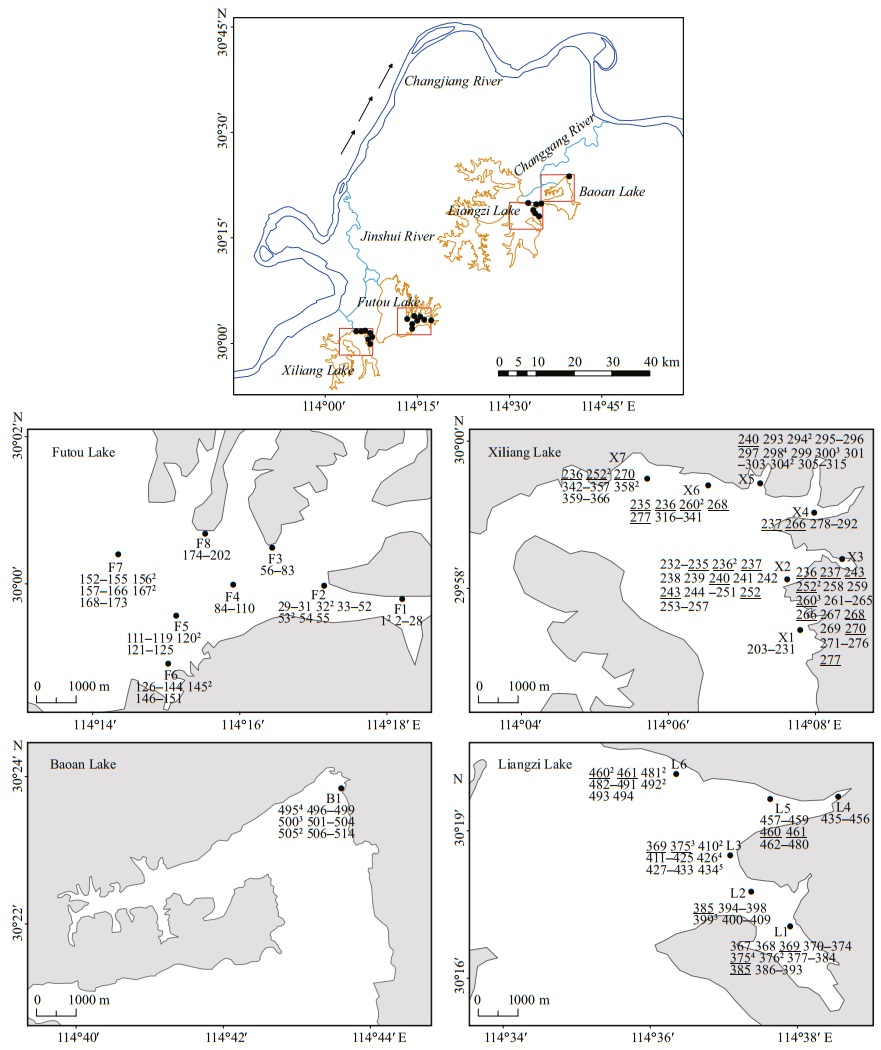
|
| Fig.1 Location of the sampling lakes of Vallisneria natans (the arrows indicate the direction of water flow) and distribution of multilocus genotypes (MLGs) in each subpopulation Samples belonging to the same MLGs are represented by the same normal numbers among subpopulations. Numbers in superscript are the ramet number of each MLG in each subpopulation. Normal numbers underlined denote MLGs shared between two or more subpopulations. |

|
Historically, the above four lakes naturally connected with the mainstream of the Changjiang River and lake water level fluctuated seasonally with the rise and fall of the Changjiang River. To reduce flooding around the lakes, sluice gates were built in the estuaries of the Changgang and Jinshui Rivers in 1926 and 1935, respectively (Committee for Lake Records of Hubei Province, 2014) (Table 1). Since then, the four lakes have been disconnected from the Changjiang River, resulting in the termination of natural seasonal lake water fluctuations. In the decades that followed, to better regulate the water level in each lake, the direct waterway connection between the neighboring lakes was cut off and more sluice gates were built on the rivers connected to each of them, resulting in each lake becoming isolated and the amplitude of water level fluctuation largely reduced.
Since the sluice gates were built and water level artificially regulated, exploitation and use of the lake resources by humans have increased greatly, resulting in an increase in the deterioration of water quality and in the intensity of mechanical disturbance in the lakes.The extent of human disturbance is severe in the Futou and Baoan Lakes, but relatively mild in the Xiliang and Liangzi lakes (Jin et al., 1999; Peng et al, 2004; Yang et al., 2017) (Table 1). In Baoan Lake, some submerged plants had disappeared at the local scale, so some recovery measurements, such as planting materials originating from the own lake, were conducted at the end of the 1990s (Jin et al., 1999). Relatively, the other lakes experienced recovery cultivation at much smaller scales. Perhaps, the plant genetic variation in the Baoan Lake may not be as natural as that in the other three lakes. Therefore, this study mainly focused on the plant samples from the three lakes but the Baoan Lake.
Vallisneria natans (Lour.) H. Hara (Hydrocharitaceae), native to China, is a cosmopolitan submerged species, found especially in the tropical and subtropical zones (Wang et al., 2010; Zhou et al., 2016). This plant is one of the dominant species in natural submerged communities (Chen et al., 2008; Wang et al., 2010) and one of the pioneer species in the restoration of degraded ecosystems (Xie et al., 2007) in the lakes of the Changjiang River Basin. V. natans has a rosette growth form with multiple belt-like leaves and thrives in clear waters (Bornette and Puijalon, 2010). Decreasing light can lower the survival rates of V. natans seedlings (Zhou et al., 2016), resulting from a possible sensitive response to changes in water depth and quality. V. natans is a dioecious ephydrophilous species (pollination occurs on the surface of the water) (Zhou et al., 2016) and its sexual reproduction is greatly dependent on aquatic environmental conditions. Different aquatic environmental characteristics may favor different alleles in different populations (Slatkin, 1987), hence increasing genetic differentiation. Conversely, similar aquatic environmental conditions and shared selection pressure might facilitate the production of genetically similar populations (Turner et al., 2018). V. natans overwinters mainly by seeds and reproduces mainly by sexual seedling in the middle-lower reaches of the Changjiang River (Chen et al., 2008; Wang et al., 2010; Zhou et al., 2016). In the vegetative growth season, new ramets are produced through the extension of stolons. When the stolons break, vegetative propagules are produced. Therefore, dispersal via both seeds and vegetative propagules can contribute to gene flow at the subpopulation and population levels. Sluices may considerably reduce gene flow by hydrochory between lake populations, thus increasing genetic differentiation among isolated lakes. Therefore, changes in lake water environments and in hydrological connections due to human activities may simultaneously affect the genetic variation of V. natans.
The aim of our study was (1) to evaluate the genetic diversity of V. natans at the subpopulation level in each lake, (2) to identify the pair of lake populations, which has a closer genetic relationship between each other, and (3) to determine what factors (hydrological connection, human disturbance intensity or other factors) may have a relatively greater effect on genetic variation in V. natans. We expect that the results will enhance our understanding of the impact of human activities on the genetic variation of submerged plants.
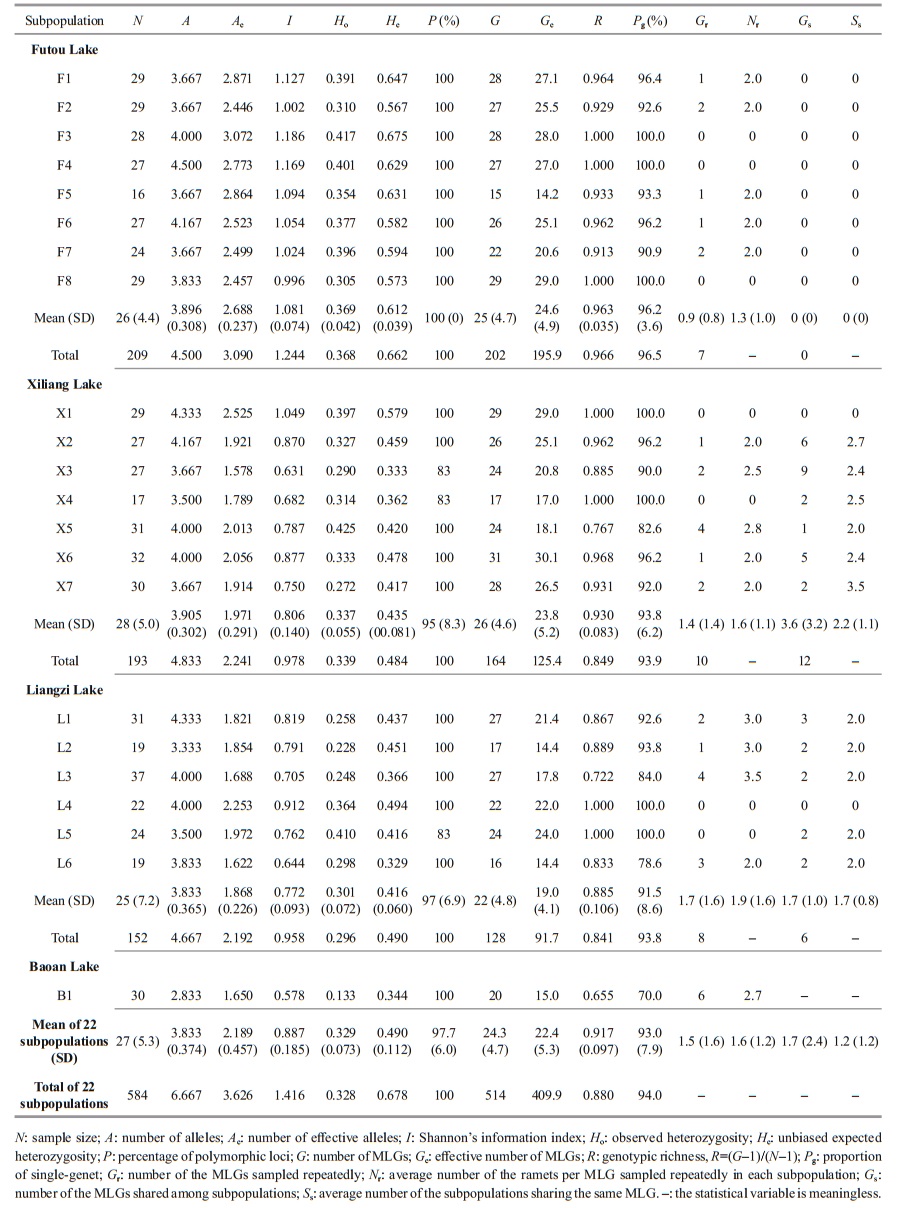
2 MATERIAL AND METHOD 2.1 Plant sampleIn June 2017, we sampled 6–8 subpopulations (152–209 individuals) of V. natans from each of the Liangzi, the Xiliang, and the Futou lakes (Fig. 1). Considering that the Baoan Lake experienced cultivation at a relatively large scale, only one subpopulation (30 individuals) was sampled from that lake. However, since the cultivated plants originated from the lake itself, the samples collected could provide some references for genetic analyses of V. natans. The distance between adjacent sampling sites (subpopulations) in each lake was approximately 2 km. Distances between samples within subpopulations were approximately 1.5–2 m. The sample sizes of the subpopulations were 16–37 (Table 2), depending on the areas being continuously covered by this species in the sample sites. In total, we collected 584 samples. One leaf, representing one sample, was taken from each plant. Leaf samples were desiccated with allochroic silica gel and stored until DNA extraction. We recorded the latitude and longitude of each sampling site using GPS. Water transparency and the concentrations of total N and total P in the lake water (Table 1) were measured. They showed the extent of human disturbance in terms of water quality when plant samples were collected.
|
Total genomic DNA was extracted from dry leaf samples following a modified cetyltrimethylammonium bromide (CTAB) protocol (Saghai-Maroof et al., 1984). Using microsatellite (simple sequence repeats, SSR) primer selection, we found that our samples could amplify bands using all the primers developed by Wang et al. (2011). However, the polymorphism of V. natans primers was lower than that of some primers developed from the congeneric species V. spinulosa Yan (Chen et al., 2006) and V. americana Michaux (Burnett et al., 2009). Therefore, we finally selected three pairs of primers from V. spinulosa (VS01, VS03, and VS29) and three pairs from V. americana (Vaam_ATG002, Vaam_AAG_X013, and Vaam_AAG_X051) (Supplementary Table S1), which could amplify highly polymorphic bands.
Amplification of SSR was carried out in 20 μL containing a mix of Taq polymerase (0.1 U/μL), dATP, dCTP, dGTP, dTTP (0.4 mmol/L each), and a buffer (ComWin Biotech, Beijing, China). PCRs were performed in a C1000 Thermal Cycler (Bio-Rad Laboratories, Hercules, CA, USA). A denaturation period of 5 min at 95 ℃ was followed by 34 cycles of 50 s at 94 ℃, 50 s at optimum annealing temperature, 60 s at 72 ℃, and for final extension, 10 min at 72 ℃. PCR products were analyzed by Tianyi Huiyuan Biotechnology Co. Ltd. (Wuhan, China), and the length of DNA fragments was determined using GeneMarker software (version 1.3; SoftGenetics, State College, PA, USA).
2.3 Data analysesVallisneria natans is a diploid species. The DNA fragment lengths of the amplification products were used. Analyses of genetic diversity (A, number of alleles; Ae, effective number of alleles; I, Shannon's information index; Ho, observed heterozygosity; He, unbiased expected heterozygosity; P, percentage of polymorphic loci, allele frequency), unbiased genetic identity, principal coordinate analyses (PCoA), and analyses of molecular variance (AMOVA) were performed using GenAlEx 6.5 (Peakall and Smouse, 2012). The highest allele frequency per locus per subpopulation was more than 25%, so the allele frequencies ≥25% were considered as dominant allele frequencies. Pairwise genetic differentiation (FST) were calculated using the values of the variance component estimates (CV) in AMOVA. Nm, the theoretical number of migrants per generation, was calculated using the equation Nm=[(1/FST)–1]/4. Although estimation of Nm via FST is now generally considered problematic, FST measures may provide reasonable estimates of Nm in cases where the spatial scale is small (Whitlock and McCauley, 1999). Therefore, among subpopulations within each lake, Nm was used to estimate gene flow.
The genetic identity matrix of subpopulations was used for a cluster analysis according to the upweighted pair-group method with arithmetic averages (UPGMA) using NTSYSpc 2.0 (Rohlf, 1998). Scatter diagrams from the principal coordiate analysis (PCoAs) of individual samples were drawn in SigmaPlot 10.0 (Systat Software Inc., San Jose, CA, USA).
Individuals with the same multilocus genotype (MLG) were treated as a clone (genet). Clone assignment and clonal diversity analysis (G, number of MLGs; Ge, effective number of MLGs) were performed using GenoType and GenoDive (Meirmans and Van Tienderen, 2004). We calculated the other parameters of clonal diversity: R, genotypic richness, where R=(G–1)/(N–1) and N is the number of samples (Dorken and Eckert, 2001); Pg, proportion of single-genet; Gr, number of the MLGs sampled repeatedly; Nr, average number of ramets per MLG sampled repeatedly in each subpopulation; Gs, number of MLGs shared among subpopulations; Ss, average number of subpopulations sharing the same MLG.
Bayesian cluster analyses of population structure were performed using STRUCTURE 2.3 (Pritchard et al., 2000) to determine the number of genetic clusters, using the admixture model with independent allele frequencies. We tested K in 10 independent runs from 1 to 10 without using sampling location as a prior to assessing convergence of lnP(D) (10 000 burn-in and 100 000 Markov Chain Monte Carlo replicates in each run). We uploaded the files of the project data to the website of Structure Harvester (http://taylor0.biology.ucla.edu/structureHarvester/, June 9, 2020) to obtain the distribution of ΔK as a function of K (Earl and vonHoldt, 2012). The best number of clusters was determined based on the ΔK method (Evanno et al., 2005).
3 RESULT 3.1 Genetic diversity and clonal diversityGenerally, the average values of expected heterozygosity (He) and genotypic richness (R) in all subpopulations studied were 0.490 and 0.917, respectively (Table 2). Among the lakes studied, the genetic diversity, at the subpopulation level, was the highest in the Futou Lake (mean Ae=2.688, I=1.081, He=0.612) and lower in both the Xiliang Lake (mean Ae=1.971, I=0.806, He=0.435) and the Liangzi Lake (mean Ae=1.868, I=0.772, He=0.416). Only one subpopulation of Baoan Lake had a very low level of genetic diversity (Ae=1.650, I=0.578, He=0.344). The total genetic diversity of all subpopulations studied in each lake showed the same trend as the average subpopulation genetic diversity (Futou Lake, total He=0.662; Xiliang Lake, total He=0.484; Liangzi Lake, total He=0.490).
The average clonal diversity, at the subpopulation level, was considerably higher in the Futou Lake (mean Ge=24.6, R=0.963) and the Xiliang Lake (mean Ge=23.8, R=0.930), compared to that in the Liangzi Lake (mean Ge=19.0, R=0.885) (Table 2). Differently, the total clonal diversity of all subpopulations in the Xiliang Lake (total R=0.849) was similar to that in the Liangzi Lake (total R=0.841), which was considerably lower than that in the Futou Lake (total R=0.966). Only one subpopulation of Baoan Lake had relatively very low clonal diversity (R=0.655).
The number of the MLGs sampled repeatedly and the average number of ramets per MLG sampled repeatedly was the highest in the subpopulations of the Liangzi Lake (mean Gr=1.7, Nr=1.9), medium in the Xiliang Lake (mean Gr=1.4, Nr=1.6), and the lowest in the Futou Lake (mean Gr=0.9, Nr=1.3) (Fig. 1, Table 2). The proportion of single-genet (Pg) was in the converse order to the values of Gr and Nr. The number of MLGs shared among subpopulations and the average number of subpopulations sharing the same MLG was the highest in the Xiliang Lake (mean Gs=3.6, Ss=2.2), but no MLGs were shared among the Futou Lake subpopulations (Fig. 1).
3.2 Genetic variation among subpopulations within each lakeThe results of AMOVA showed that the genetic variation was much less among subpopulations (8%– 18%) than within subpopulations in each lake, especially low among the subpopulations of the Futou and Xiliang Lakes (Table 3). Therefore, the values of Nm, calculated on the basis of subpopulation differentiation, were higher in the Futou Lake (2.876) and the Xiliang Lake (2.313), compared to the Liangzi Lake (1.142).
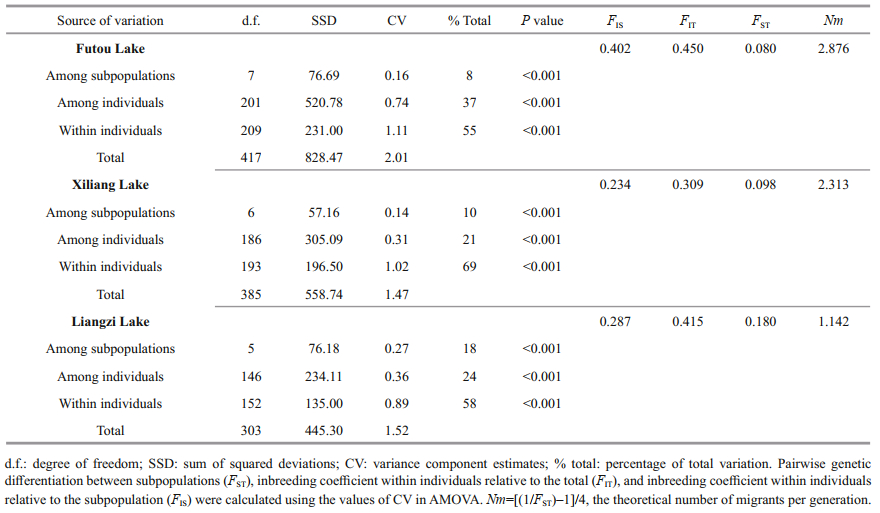
|
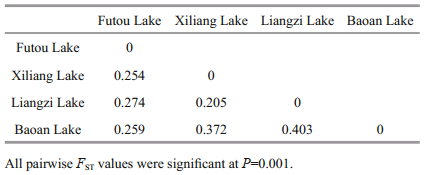
The values of pairwise genetic differentiation among lake populations (FST) ranged from 0.205 to 0.403 (Table 4). Based on genetic identity, the subpopulations originating from each lake were clustered together (Fig. 2). Among the four lake populations, the subpopulations from the Xiliang Lake and from the Liangzi Lake had relatively close genetic relationships, while only one subpopulation of the Baoan Lake was clustered together with the Futou Lake subpopulations.
|

|
| Fig.2 Dendrograms of subpopulations of Vallisneria natans from four lakes using Nei's unbiased genetic identity coefficients F1–F8, Futou Lake; X1–X7, Xiliang Lake; L1–L6, Liangzi Lake; B1, Baoan Lake. |
According to the PCoA results, the individuals originating from each lake tended to have close genetic relationships (Fig. 3). Along axis one, which explained 29.7% of the total genetic variation, the individuals from the Futou Lake (severely disturbed) were different from those of the Liangzi and Xiliang Lakes (mildly disturbed). All individuals from the Baoan subpopulation were more or less within the range of Futou Lake individuals.

|
| Fig.3 Scatter plots of principle coordinate analyses (PCoA) for individuals of Vallisneria natans in four lakes Subpopulation F1–F8, Futou Lake; X1–X7, Xiliang Lake; L1–L6, Liangzi Lake; B1, Baoan Lake. |
The Bayesian cluster analyses suggested K=2 as the optimal number of clusters based on the value of ΔK (Supplementary Fig.S1). The individuals of the Xiliang and Liangzi Lakes mainly belonged to a genetic cluster different from those of the Futou and Baoan Lakes (Fig. 4). The result of K=3 was also presented due to the high value of ΔK. When K=3, the individuals of the Futou and Baoan Lakes still belonged to the same cluster, but those of the Xiliang Lake were genetically differentiated from those of the Liangzi Lake.
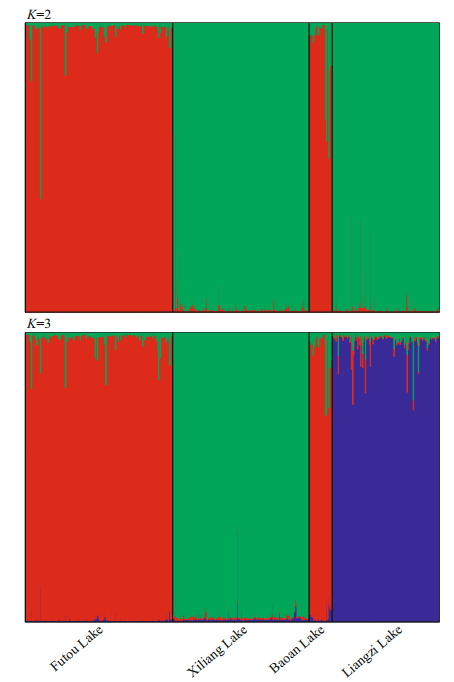
|
| Fig.4 Estimated genetic structure of Vallisneria natans populations in four lakes, inferred by a Markov chain Monte Carlo clustering (STRUCTURE) at the individual level Black lines indicate different population origins. |
The analyses of the allele frequency showed that the dominant allele frequencies (≥25%) were generally similar among the subpopulations within each lake (Table 5). Among the four lakes, the dominant allele frequencies of Xiliang subpopulations exhibited a similarity to those from the Liangzi Lake at some loci, e.g. VS01, VS03 and Vaam_AAG_ X013. Additionally, the Liangzi population had the most private alleles.
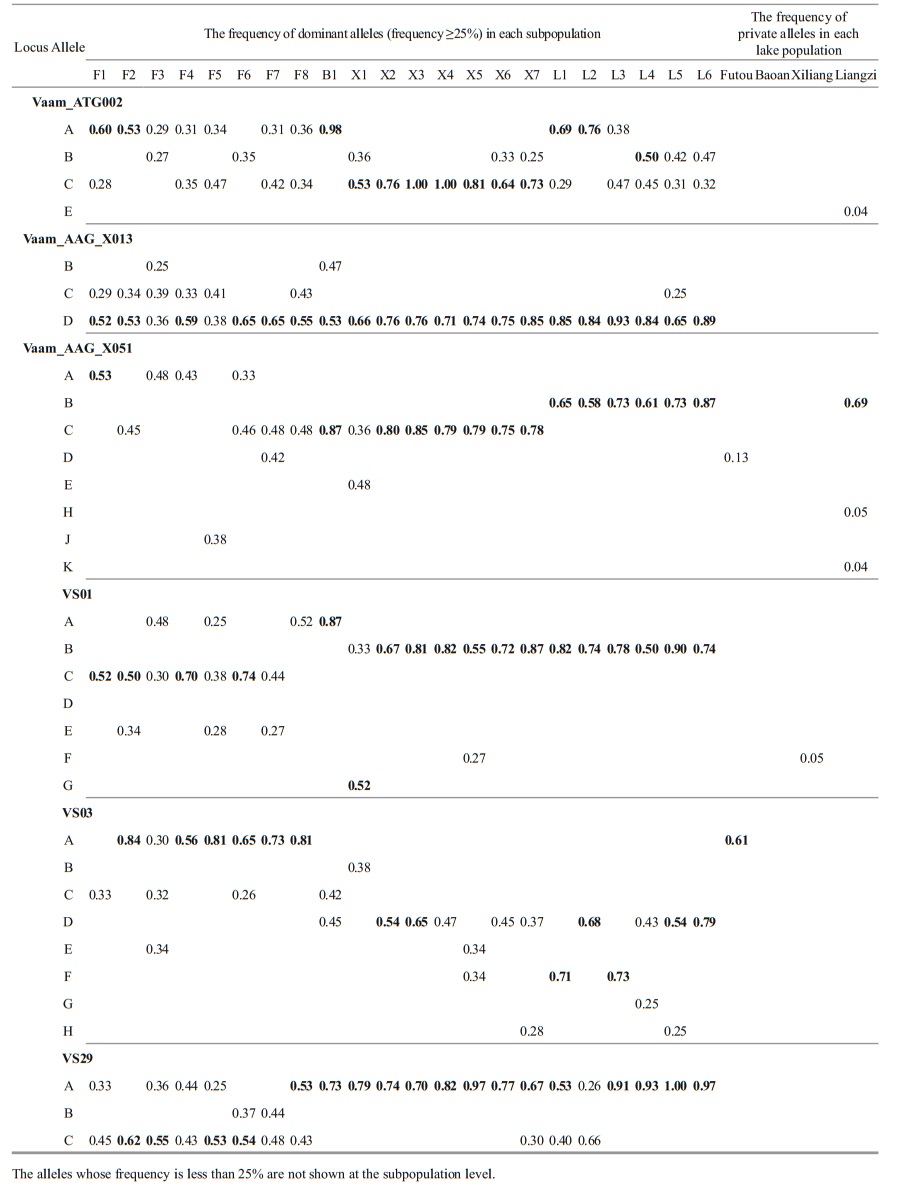
|
The values of genetic diversity for all subpopulations of V. natans studied in the present study (mean He=0.490, total He=0.678, 584 individuals) were higher than those detected in 196 individuals from six lake populations of this species (mean He=0.24, total He=0.32) in the middle-lower reaches of the Changjiang River using inter-simple sequence repeat (ISSR) primers (Wang et al., 2010). The difference in values may be related to the higher variability of SSR markers used in the present work than when using ISSR markers, in addition to our larger sample size. Compared with the congeneric V. americana in Chesapeake Bay (mean He=0.53, mean R=0.57) (Lloyd et al., 2011), V. natans in the four lakes studied in this work had slightly lower genetic diversity but higher clonal diversity for all subpopulations (mean R=0.917). Lloyd et al. (2011) used the same type of molecular markers (SSR) and a comparable sample size (mean N=26) as our study (mean N=27) but larger distances between individual samples (5-10 m) within each site. The high clonal diversity in this study indicates smaller clone size and less vigorous clone growth in V. natans than in V. americana. The limited clone growth of V. natans agrees with the previous finding that in natural populations only a low proportion of V. natans plants can produce a few ramets (Chen et al., 2008).
In the Futou Lake, which was severely disturbed by humans, V. natans exhibited the highest genetic diversity at the subpopulation (mean He=0.612) and the total lake population (total He=0.662) levels among the four lakes. However, the genetic diversity of V. natans was low but similar in the Xiliang (mean He=0.435, total He=0.484) and the Liangzi (mean He=0.416, total He=0.490) Lakes, and both were affected by mild disturbance. This result is consistent with the influence of environmental disturbances on the seagrass Zostera marina as reported by Kim et al. (2019), where the highest genetic diversity was detected in the population with the greatest disturbances. In fact, with disturbances, especially anthropogenic ones, the general plant response is an increase in sexual reproductive effort, thus increasing genetic diversity to confer higher resilience to plants under unfavorable conditions (Reusch, 2006; Cabaco and Santos, 2012; Kim et al., 2019). The contribution of sexual reproduction of V. natans in the severely disturbed Futou Lake is supported by very few MLGs sampled repeatedly (mean Gr=0.9), a high proportion of single-genet (mean Pg=96.2%), and high genotypic richness (mean R=0.963) in the subpopulations. When collecting samples, we observed that V. natans had a lower plant density and produced fewer ramets in the Futou Lake compared to the Xiliang and Liangzi Lakes. Relatively stable aquatic environments in mildly disturbed lakes facilitate the production of more ramets, such as seagrass Z. Marina (Kim et al., 2019). In contrast, unstable environments in the severely disturbed lake repress the formation of dense ramets but provide opportunities for seedling recruitment (Reusch, 2006), if the aquatic environment can meet the growth and reproduction needs of V. natans. Additionally, the diversity level differences between the populations might be affected by recovery cultivation, but the effect may be limited to a very small extent. In the Futou Lake, strong gene flow (Nm=2.876) among subpopulations may also be a contributor to the high genetic diversity. Moreover, gene exchanges mainly depend on the movement of sexual propagules and even pollens in terms of no MLGs shared (Gs=0) among subpopulations in that lake. As an annual plant in the region studied (Chen et al., 2008), V. natans overwinters by seeds and can produce a great quantity of seeds (321 seeds per fruit, 5 fruits per female flowering ramet, Zhou et al., 2016). Fruits of Vallisneria species can float in water after detachment from the mother plants (Liu et al., 2005) and hence, offering a wider dispersal range. Being a species exhibiting epihydrophily, the large number of pollen produced by V. natans also contribute to the species' dispersal through water by migrating within the populations (Wang et al., 2010), although the distances travelled by the pollen are limited to several meters (Lloyd et al., 2018). However, Vallisneria fruits contain seeds sired by many different fathers (seven fathers on average) (Lloyd et al., 2018) and contribute to increasing genetic diversity at the subpopulation level.
In the mildly disturbed Liangzi Lake, V. natans had considerably low clonal diversity at the subpopulation (mean R=0.885) and total lake population (total R=0.841) levels, compared to the Futou Lake. V. natans plants from the Liangzi Lake had larger clone sizes (Gr=1.7, Nr=1.9) and a lower proportion of single-genet (Pg=91.5%) than those from the Futou Lake. In less disturbed environments, submerged V. natans plants produce larger clones and more ramets, like the seagrass Enhalus acoroides. In a study carried out by Yu et al. (2019), E. acoroides had the largest clone with only three ramets in a seriously disturbed plot, while in a less disturbed plot, 10.9% of the genets contained more than three ramets and the largest clone covered about 15 m2. Large clones may decrease the opportunity for seedling recruitment, thus decreasing genetic diversity within populations, reducing gene flow (Nm=1.142 in the Liangzi Lake), and enlarging differentiation among subpopulations to a certain degree.
The clonal diversity of V. natans in the Xiliang Lake (mean R=0.930, total R=0.849) was almost as high as that in the Futou Lake at the subpopulation level, but as low as that in the Liangzi Lake (total R=0.841) at the total lake population level. The difference in genotypic richness between the two levels in the Xiliang Lake may be explained by relatively strong gene exchanges via vegetative propagules among subpopulations (mean Gs=3.6, Ss=2.2, Nm=2.313). Although V. natans does not produce overwintering turions (Chen et al., 2008; Zhou et al., 2016), ramets produced by stolon disruption in the vegetative growth season can migrate via hydrochory within populations.
Only one subpopulation of V. natans in the Baoan Lake had very low genetic (He=0.344) and clonal (R=0.655) diversity and a very low proportion of single-genet (Pg=70%), indicating that restoration experience may decrease genetic diversity at a subpopulation level.
Therefore, under severely disturbed conditions, V. natans populations are maintained mainly by sexual reproduction through the production of genetically new individuals and continuous seedling recruitment, while under relatively mildly disturbed conditions, the contribution of vegetative reproduction plays an ineligible role in population dynamics and expansion, consistent with the performance of some seagrass species (Cabaco and Santos, 2012; Kim et al., 2019). Consequently, human disturbance might increase the genetic diversity of V. natans at the subpopulation level to a certain extent.
4.2 Genetic relationships among lake populationsBased on the genetic identity at the subpopulation level and the PCoA and Bayesian cluster analyses at the individual level, the samples originating from each lake were clustered together, implying genetic differentiation occurring among lake populations of V. natans. This tendency was also supported by the analyses of dominant allele frequency. The result is consistent with other studies on genetic variation in natural populations of submerged plants (Li et al., 2004; Wang et al., 2010; Han et al., 2014; Cao et al., 2017). As Laushman (1993) considered, lake habitats of submerged plants are ‘islands' in a terrestrial ‘sea', so little gene exchange would be expected between the habitats, and founder effects and genetic drift would be more pronounced under such conditions. Therefore, a great isolation may be observed among freshwater lake populations relative to most terrestrial species and coastal species (Laushman, 1993; Reusch, 2002; Yu et al., 2019).
Among the lakes, the subpopulations of V. natans from the Xiliang and the Liangzi Lakes, disturbed to a similarly mild extent, had a relatively close genetic relationship. Only one subpopulation of V. natans from the Baoan Lake was clustered together with those of the Futou Lake, which was severely disturbed. Furthermore, at the individual level, Bayesian cluster analyses showed a similar trend (K=2). The results indicate that the intensity of human disturbance might differentiate lake populations of V. natans under similar climatic conditions. Different intensities of disturbance in lake environments might produce different selective pressures on submerged plants; that is to say, the populations in the relatively mildly disturbed lakes (Xiliang and Liangzi Lakes) differentiate genetically from those in severely disturbed lakes (Futou and Baoan Lakes). For instance, nutrient contents in water can impact the competitive capability among plants (Bornette and Puijalon, 2010), and high nutrient levels in severely disturbed lakes might favor competitive clones while low nutrient levels in mildly disturbed lakes might favor tolerators. Natural selection favoring adaptations to local environmental conditions leads to genetic differentiation of local populations (Slatkin, 1987). This selective force due to different extents of human disturbance seems to be so strong that it outperforms the force of historical and current gene flow tending to produce genetic homogeneity (Slatkin, 1987) between neighboring lake populations with direct interconnecting waterways (Committee for Lake Records of Hubei Province, 2014). In addition, gene flow by bird-mediated dispersal might contribute to genetic relationships among freshwater populations (Chen et al., 2009; Cao et al., 2017). The close plant genetic relationship between the Liangzi and Xiliang Lakes might benefit from more frequent bird inter-migration, for the two lakes; more diverse aquatic vegetation attracts and sustains more birds (Peng et al., 2004; Yang et al., 2017).
The FST values between the neighboring lake populations were more than 0.25 (0.254 between the Futou and Xiliang Lakes; 0.403 between the Liangzi and Baoan Lakes), indicating very large differentiation between the historically directly interconnected lakes (Wright, 1978). Our observed pairwise population differentiation (FST 0.205-0.403) was higher than that estimated by Wang et al. (2010) for V. natans (FST=0.132) and V. spinulosa (FST=0.202), and by Chen et al. (2007) for V. spinulosa (FST=0.06), all of which were sampled from lakes of the middle-lower reaches of the Changjiang River. The increase in FST values over time might be related to the growing reduction of hydrological connectivity (Chen et al., 2009) between the sampled lakes. Wang et al. (2010) also considered that more divergence among Vallisneria populations would occur with further reduction in connectivity. Similar to the findings of Wang et al. (2010), no widespread MLG shared among lakes was detected in the present study, further suggesting that water-dispersed propagules are rarely transferred between populations and the linkage by hydrological connection between populations is becoming weak.
5 CONCLUSIONOur results showed that for freshwater-submerged V. natans, human disturbance decreased plant density and clonal growth but might increase the genetic and clonal diversity at a subpopulation level and enhance gene flow among subpopulations by sexual propagule movement. Under similar climatic conditions, different intensities of disturbance seem to have such a high selective potential to differentiate genetically adjacent lake populations that they outperform the forces of gene flow through historical direct hydrological interconnection, which tends to produce genetic homogeneity. Our findings imply that human disturbance has a profound effect on the evolutionary process of natural populations of submerged plants. Increased subpopulation genetic diversity, especially by increasing sexual reproduction, can enhance resistance and resilience to environmental disturbances because subpopulations that are repeatedly damaged due to disturbance can rapidly reestablish sexually via seed germination. To a certain degree, we could expect that disturbed populations have the possibility of restoring spontaneously if humans cease to perturb natural ecosystems in the future.
6 DATA AVAILABILITY STATEMENTData of microsatellite (SSR) primer pairs used for DNA amplification in this study and the value of ΔK in Bayesian cluster analyses (STRUCTURE) are included in the supplementary information files of this published article. Data on allele information of Vallisneria natans, generated and analyzed during the current study, are available from the corresponding author upon reasonable request.
Electronic supplementary materialSupplementary material (Supplementary Table S1 & Fig.S1) is available in the online version of this article at https://doi.org/10.1007/s00343-020-0277-1.
Bornette G, Puijalon S. 2010. Response of aquatic plants to abiotic factors: a review. Aquatic Sciences, 73(1): 1-14.
DOI:10.1007/s00027-010-0162-7 |
Burnett R K Jr, Lloyd M W, Engelhardt K A M, Neel M C. 2009. Development of 11 polymorphic microsatellite markers in a macrophyte of conservation concern, Vallisneria americana Michaux (Hydrocharitaceae). Molecular Ecology Resources, 9(5): 1427-1429.
DOI:10.1111/j.1755-0998.2009.02578.x |
Cabaco S, Santos R. 2012. Seagrass reproductive effort as an ecological indicator of disturbance. Ecological Indicators, 23: 116-122.
DOI:10.1016/j.ecolind.2012.03.022 |
Cao Q J, Mei F F, Wang L. 2017. Population genetic structure in six sympatric and widespread aquatic plants inhabiting diverse lake environments in China. Ecology and Evolution, 7(15): 5713-5723.
DOI:10.1002/ece3.3141 |
Chambers P A, Lacoul P, Murphy K J, Thomaz S M. 2007. Global diversity of aquatic macrophytes in freshwater. Hydrobiologia, 595(1): 9-26.
DOI:10.1007/s10750-007-9154-6 |
Chen L, Les D H, Xu L M, Yao X H, Kang M, Huang H W. 2006. Isolation and characterization of a set of microsatellite loci in the submerged macrophyte, Vallisneria spinulosa Yan (Hydrocharitaceae). Molecular Ecology Notes, 6(4): 1243-1245.
DOI:10.1111/j.1471-8286.2006.01503.x |
Chen L, Xu L M, Huang H W. 2007. Genetic diversity and population structure in Vallisneria spinulosa (Hydrocharitaceae). Aquatic Botany, 86(1): 46-52.
DOI:10.1016/j.aquabot.2006.09.001 |
Chen L, Ye Q G, Pan L Z, Xu L M, Huang H W. 2008. Vallisneria species in lakes of the middle-lower reaches of the Yangtze River of China. Journal of Plant Ecology, 32(1): 106-113.
(in Chinese with English abstract) DOI:10.3773/j.issn.1005-264x.2008.01.012 |
Chen Y Y, Li X L, Yin L Y, Cheng Y, Li W. 2009. Genetic diversity and migration patterns of the aquatic macrophyte Potamogeton malaianus in a potamo-lacustrine system. Freshwater Biology, 54(6): 1178-1188.
DOI:10.1111/j.1365-2427.2008.02165.x |
Committee for Lake Records of Hubei Province. 2014. Lakes Records of Hubei Province. Hubei Science and Technology Press, Wuhan, China.
(in Chinese)
|
Dorken M E, Eckert C G. 2001. Severely reduced sexual reproduction in northern populations of a clonal plant, Decodon verticillatus (Lythraceae). Journal of Ecology, 89(3): 339-350.
DOI:10.1046/j.1365-2745.2001.00558.x |
Earl D A, vonHoldt B M. 2012. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources, 4(2): 359-361.
DOI:10.1007/s12686-011-9548-7 |
Evanno G, Regnaut S, Goudet J. 2005. Detecting the number of clusters of individuals using the software structure: a simulation study. Molecular Ecology, 14(8): 2611-2620.
DOI:10.1111/j.1365-294X.2005.02553.x |
Han Q X, Wang G X, Li W, Liu F. 2014. Genetic diversity of Potamogeton pectinatus L. in relation to species diversity in a pair of lakes of contrasting trophic levels. Biochemical Systematics and Ecology, 57: 60-66.
DOI:10.1016/j.bse.2014.07.003 |
Jin G, Li Z J, Liu H Q, Wen Z R, Chen H D. 1999. Recovery of submerged vegetation and its fishery benefit in Bao'an Lake. Journal of Lake Sciences, 11(3): 260-266.
(in Chinese with English abstract) DOI:10.18307/1999.0311 |
Kim Y K, Kim S H, Yi J M, Park S R, Lee K S. 2019. Influence of environmental disturbances and reproductive strategy on genetic diversity and differentiation of Zostera marina populations on the southern coast of Korea. Marine Ecology, 40(1): e12537.
DOI:10.1111/maec.12537 |
Lacoul P, Freedman B. 2006. Environmental influences on aquatic plants in freshwater ecosystems. Environmental Reviews, 14(2): 89-136.
DOI:10.1139/a06-001 |
Laushman R H. 1993. Population genetics of hydrophilous angiosperms. Aquatic Botany, 44(2-3): 147-158.
DOI:10.1016/0304-3770(93)90069-9 |
Li W, Xia L Q, Li J Q, Wang G X. 2004. Genetic diversity of Potamogeton maackianus in the Yangtze River. Aquatic Botany, 80(4): 227-240.
DOI:10.1016/j.aquabot.2004.07.003 |
Liu G H, Zhou J, Li W, Cheng Y. 2005. The seed bank in a subtropical freshwater marsh: implications for wetland restoration. Aquatic Botany, 81(1): 1-11.
DOI:10.1016/j.aquabot.2004.07.001 |
Lloyd M W, Burnett R K Jr, Engelhardt K A M, Neel M C. 2011. The structure of population genetic diversity in Vallisneria americana in the Chesapeake Bay: implications for restoration. Conservation Genetics, 12(5): 1269-1285.
DOI:10.1007/s10592-011-Lloyd |
Lloyd M W, Tumas H R, Neel M C. 2018. Limited pollen dispersal, small genetic neighborhoods, and biparental inbreeding in Vallisneria americana. American Journal of Botany, 105(2): 227-240.
DOI:10.1002/ajb2.1031 |
Meirmans P G, Van Tienderen P H. 2004. GENOTYPE and GENODIVE: two programs for the analysis of genetic diversity of asexual organisms. Molecular Ecology Notes, 4(4): 792-794.
DOI:10.1111/j.1471-8286.2004.00770.x |
Nilsson C, Brown R L, Jansson R, Merritt D M. 2010. The role of hydrochory in structuring riparian and wetland vegetation. Biological Reviews, 85(4): 837-858.
DOI:10.1111/j.1469-185X.2010.00129.x |
Peakall R, Smouse P E. 2012. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics, 28(19): 2537-2539.
DOI:10.1093/bioinformatics/bts460 |
Peng Y H, Jian Y X, Wang J B, Ni L Y. 2004. A comparative study on aquatic plant diversity in five largest lakes of Hubei Province in China. Acta Hydrobiologica Sinica, 28(5): 464-470.
(in Chinese with English abstract) DOI:10.3321/j.issn:1000-3207.2004.05.002 |
Pritchard J K, Stephens M, Donnelly P. 2000. Inference of population structure using multilocus genotype data. Genetics, 155(2): 945-959.
|
Qin B Q. 2002. Approaches to mechanisms and control of eutrophication of shallow lakes in the middle and lower reaches of the Yangze River. Journal of Lake Sciences, 14(3): 193-202.
(in Chinese with English abstract) DOI:10.18307/2002.0301 |
Rejmánková E. 2011. The role of macrophytes in wetland ecosystems. Journal of Ecology and Field Biology, 34(4): 333-345.
DOI:10.5141/JEFB.2011.044 |
Reusch T B H. 2002. Microsatellites reveal high population connectivity in eelgrass (Zostera marina) in two contrasting coastal areas. Limnology and Oceanography, 47(1): 78-85.
DOI:10.4319/lo.2002.47.1.0078 |
Reusch T B H. 2006. Does disturbance enhance genotypic diversity in clonal organisms? A field test in the marine angiosperm Zostera marina. Molecular Ecology, 15(1): 277-286.
DOI:10.1111/j.1365-294X.2005.02779.x |
Rohlf F J. 1998. NTSYS-pc. Numerical taxonomy and multivariate analysis system, version 2.02. Exeter Software, Setauket, New York.
|
Saghai-Maroof M A, Soliman K M, Jorgensen R A, Allard R W. 1984. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proceedings of the National Academy of Sciences of the United States of America, 81(24): 8014-8018.
DOI:10.1073/pnas.81.24.8014 |
Slatkin M. 1987. Gene flow and the geographic structure of natural populations. Science, 236(4803): 787-792.
DOI:10.1126/science.3576198 |
Turner C B, Marshall C W, Cooper V S. 2018. Parallel genetic adaptation across environments differing in mode of growth or resource availability. Evolution Letters, 2(4): 355-367.
DOI:10.1002/evl3.75 |
Wang B, Liao H, Zhao Y, Li W, Song Z P. 2011. Microsatellite loci in Vallisneria natans (Hydrocharitaceae) and cross-reactivity with V. spinulosa and V. denseserrulata. American Journal of Botany, 98(3): e44-e47.
DOI:10.3732/ajb.1000441 |
Wang B, Song Z P, Liu G H, Lu F, Li W. 2010. Comparison of the extent of genetic variation of Vallisneria natans and its sympatric congener V. spinulosa in lakes of the middle-lower reaches of the Yangtze River. Aquatic Botany, 92(4): 233-238.
DOI:10.1016/j.aquabot.2009.12.006 |
Wang S M, Dou H S. 1998. Memoirs of Lakes in China. Science Press, Beijing, China.
(in Chinese)
|
Whitlock M C, McCauley D E. 1999. Indirect measures of gene flow and migration: FST≠1/(4Nm+1). Heredity, 82(2): 117-125.
DOI:10.1038/sj.hdy.6884960 |
Wright S. 1978. Evolution and the Genetics of Populations. Volume 4: Variability Within and Among Natural Populations. The University of Chicago Press, Chicago.
|
Xie Y H, Deng W, Wang J D. 2007. Growth and root distribution of Vallisneria natans in heterogeneous sediment environments. Aquatic Botany, 86(1): 9-13.
DOI:10.1016/j.aquabot.2006.08.002 |
Yang J F, Du D, Tian S S, Dong W L, Yang X, Min S F. 2017. Biodiversity assessment of typical lake wetlands in Hubei Province. Journal of Hydroecology, 38(3): 15-22.
(in Chinese with English abstract) DOI:10.15928/j.1674-3075.2017.03.003 |
Yu S, Wu Y C, Serrao E A, Zhang J P, Jiang Z J, Huang C, Cui L J, Thorhaug A, Huang X P. 2019. Fine-scale genetic structure and flowering output of the seagrass Enhalus acoroides undergoing disturbance. Ecology and Evolution, 9(9): 5186-5195.
DOI:10.1002/ece3.5106 |
Zhou Y, Li X J, Zhao Y, Zhou W, Li L, Wang B, Cui X H, Chen J K, Song Z P. 2016. Divergences in reproductive strategy explain the distribution ranges of Vallisneria species in China. Aquatic Botany, 132: 41-48.
DOI:10.1016/j.aquabot.2016.04.005 |
 2021, Vol. 39
2021, Vol. 39


