Institute of Oceanology, Chinese Academy of Sciences
Article Information
- JIANG Shan, JIN Jie, ZHANG Guosen, CHANG Yan, ZHANG Zhaoru, ZHOU Meng, WANG Xiaolu, ZHANG Jing, WU Ying
- Nitrate in the Changjiang diluted water: an isotopic evaluation on sources and reaction pathways
- Journal of Oceanology and Limnology, 39(3): 830-845
- http://dx.doi.org/10.1007/s00343-020-0149-8
Article History
- Received Apr. 7, 2020
- accepted in principle May. 18, 2020
- accepted for publication Jul. 14, 2020
2 School of Oceanography, Shanghai Jiao Tong University, Shanghai 200240, China
Nitrogen (N) is essential in the biosynthesis of DNA, protein, phospholipid, etc., with global storage of 2.2×1010 Tg (Kuypers et al., 2018). Dissolved inorganic nitrogen (DIN) accounts for a small portion of total N inventory in the biosphere (Kuypers et al., 2018), but it is characterized by active cycling and transport. Biological and industrial nitrogen fixation introduces ca. 425 Tg N-DIN per year into the biosphere; while only 300 Tg N-DIN per year can be transformed to dinitrogen gas (Kuypers et al., 2018). Excessive DIN in terrestrial ecosystems, mainly nitrate (NO3ˉ), is transported by surface rivers into coastal oceans through estuaries (47.8 Tg N/a; Galloway et al., 2004). The enrichment of land-borne NO3ˉ in coastal waters frequently triggers harmful algae blooms and subsequent hypoxia, causing significant economic losses (Justić et al., 2003). Therefore, evaluating the magnitude of riverine NO3ˉ fluxes and exploring the reaction pathways related with NO3ˉ concentration variations in estuaries have received great attention from coastal managers and researchers (Wong et al., 2014; Loken et al., 2016; Yan et al., 2017; Domangue and Mortazavi, 2018).
The Changjiang River, length of ca. 6 300 km, delivers 9.24×1011 m3/a river water to adjacent coastal oceans, ranking the fifth largest rivers on a global scale (Milliman and Farnsworth, 2011). In the 1980s, the Changjiang delivered ca. 6.0×1010 mol NO3ˉ/a to the East China Sea and the Yellow Sea (Edmond et al., 1985). Afterwards, the NO3ˉ concentration in the Changjiang River water increased more than three folds, indicating a marked enhancement in riverborne NO3ˉ input. Land-use changes due to rapid urbanization, overuse of fertilizers and sewage discharge are deemed to be key contributors of riverine NO3ˉ (Dai et al., 2011). The Changjiang diluted water (CDW), consisting of the Changjiang River runoff and coastal saline water, could extend to more than 200-km distance from the river mouth and cover 104 km2 surface area (31‰ isohaline; Chang et al., 2014). A series of research projects have been launched to explore the spatial/temporal distribution of riverine NO3ˉ and reactions related to the concentration variation (Zhang, 1996; Yao et al., 2014; Yu et al., 2015; Liu et al., 2016; Yan et al., 2017). In the Changjiang estuary, nitrification, biological uptake, denitrification and anaerobic ammonium oxidation (Anammox) in the water column and/or benthic sediments have been determined (Song et al., 2013; Yan et al., 2017). These reaction pathways associated with NO3ˉ addition (nitrification) and removal (Anammox, denitrification, assimilation, etc.) fundamentally influence NO3ˉ concentration and the concentration-based impacts. Stable isotope compositions such as δ15N-NO3ˉ and δ18O-NO3ˉ are valuable for identifying reaction pathways of NO3.ˉ In estuaries, mineralization of pelagic organic matter and subsequent nitrification decreases both δ15N-NO3ˉ and δ18O-NO3.ˉ In contrast, biological consumption leads to an increase in δ15NNO3ˉ and δ18O-NO3.ˉ Theoretically, the ratio for the enhancement in δ15N-NO3ˉ and δ18O-NO3ˉ is 1꞉1 (Granger et al., 2010). Denitrification in the water column could produce a similar enrichment in δ15NNO3ˉ and δ18O-NO3ˉ; while the enhancement in the isotope compositions caused by denitrification in the benthic sediment may not be observed if NO3ˉ is fully converted (Yan et al., 2017). Accordingly, the combination of NO3ˉ concentration and its stable isotope composition could improve our understanding of NO3ˉ production and removal in the CDW.
Environmental factors in the Changjiang estuary, such as salinity, total suspended matter (TSM), and dissolved oxygen (DO), are highly dynamic because of the co-variation of tidal amplitude and the Changjiang River discharge rate, as well as stratification (Zhang, 1996). In addition, there are several outlets for the Changjiang River runoff (Fig. 1). Discharge rates, tidal amplitudes, and related water residence time among these outlets are significantly different (Wu et al., 2010), which also deeply influences the related environmental parameters in the regions outside of these outlets. Biogeochemical reactions, including nitrification, denitrification, and assimilation are sensitive to the changes in these factors (Kuypers et al., 2018). Up-tonow, the published documents only revealed the variation of NO3ˉ concentration and its isotope compositions in surface water (Liu et al., 2009) or on a single transect (Yan et al., 2017) in the Changjiang estuary. However, studies on NO3ˉ distribution and reactions, as well as dynamic linkage between environmental factors and NO3ˉ production/removal based on a comparison between multiple transects with different water depths are limited.
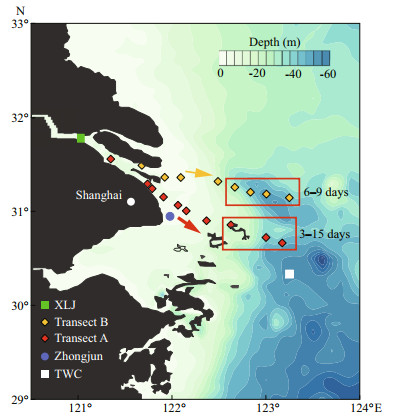
|
| Fig.1 Sampling locations along two transects in the Changjiang estuary and Zhongjun station for the gauge of tidal amplitude XLJ (Xuliujing) and TWC (Taiwan Warm Current) sites are also marked in the figure. Arrows indicate the cruise direction along two transects. Regions for diatom bloom on two transects are located in rectangles. Moreover, the water residence time in the rectangles ranged fromto 15 days and 6 to 9 days on both transects, measured by drifters at during the cruise. |
In the present study, a cruise (R/V Zheyuke Ⅱ) in theChangjiang estuary was conducted during summer 2017 when the Changjiang River discharge rate was high ((6-7)×104 m3/s gauged at Datong Station; http://www.cjh.com.cn). The objectives of this study were (1) to identify NO3ˉ sources in the Changjiang River runoff prior to river-sea mixing; (2) to determine reactions with regard to NO3ˉ production and removal along the CDW pathway; and (3) to explore the relationship between the observed reaction pathways and environmental parameters, such as salinity, DO, and TSM in the estuary.
2 MATERIAL AND METHOD 2.1 Sample collectionThe cruise in the Changjiang estuary was conducted on two transects (A and B) from 1st July to 8th July 2017, extending from river channels at the south branch (low salinity water) to the outer edge of the CDW as well as the Taiwan Warm Current (TWC) site (Fig. 1). Besides, the river water at the Xuliujing (XLJ) site was obtained on 4th July 2017 (Fig. 1). The tidal range gauged at the Zhongjun station (Fig. 1) varied from 1.8 m on 4th July to 2.4 m on 8th July 2017. Transect A is the south outlet of Changjiang River runoff and adjacent to the deep-water channel, a channel for marine transport vessels. It has been surveyed in previous research projects related to NO3ˉ transport and transformation (Wang et al., 2017; Yan et al., 2017). Transect B extends from another outlet with relatively high discharge rate. It is separated with transect A by the Changxing Island and Hengsha Island. During the survey, phytoplankton bloom (dominant species, Skeletonema costatum) was observed in the CDW outer plume on both transects (outlined in Fig. 1). The water residence in these regions ranged from 3 to 15 days, estimated by drifters released in the river mouth at the beginning of the cruise.
Water samples during the cruise were collected from a CTD rosette sampler, equipped with 12-L Niskin bottles, a conductivity-temperature-depth (SBE 911, Sea-Bird Co.) unit and a fluorescence detector. In each site, 2-4 layers of the CDW, ranging from 3- to 53-m depth, were sampled. After collection, the measurement of DO concentration was immediately conducted using a detector (JENCO®, Model 9173). Approximately 1-L water was filtered through a polycarbonate membrane (pore size: 0.4 μm, Whatman®) for the quantification of dissolved nitrogen content, δ15N-NO3ˉ and δ18O-NO3.ˉ The filtrate was stored at -20 ℃ until laboratory processing. Another fraction of water (0.3-3 L) was filtered by pre-combusted GF/F filter (average pore size: 0.7 μm, Whatman®). The solids retained on the filters were determined as the TSM concentration.
2.2 Laboratory analysisConcentrations of DIN species (NH4+, NO2ˉ, and NO3ˉ) were determined on a flow injection analyzer (Skalar Analytical B.V., The Netherlands) using standard colorimetric procedures (Hansen and Koroleff, 1999). The method determination limit for these species was approximately 0.1 μmol/L. The analytical accuracy was approximately ±3%. The concentration of total dissolved nitrogen (TDN) was determined by the potassium peroxydisulfate (K2S2O7) digestion method with the determination limit of 0.2 μmol/L and method precession of ±3% (Ebina et al., 1983). The level of dissolved organic nitrogen (DON) was obtained as the difference between TDN and DIN concentrations. The δ15N-NO3ˉ and δ18O-NO3ˉ were determined by bacterial reduction method (NO3ˉ to N2O, using Pseudomonas aureofaciens, Sigman et al., 2001) following the procedure described by Weigand et al. (2016). NO2ˉ in samples was removed by the prepared sulfanilamide solution prior to bacterial reduction (Weigand et al., 2016). The generated N2O was purified and concentrated in a Finnigan Precon System (Thermo Fisher, USA). Subsequently, N2O and trace amount of CO2 was separated by a chromatographic loop (GC Isolink; Thermo Fisher, USA). Afterwards, the δ15N and δ18O were analyzed in an isotope ratio mass spectrometer (Delta V; Thermo Fisher, USA). Measurements of δ15N were referenced to atmospheric N2 and δ18O to Vienna Standard Mean Ocean Water (VSMOW) with standards of IAEA-NO-3, USGS-34, and USGS-35. The detection limit was approximately 1 μmol/L NO3ˉ with the analytical precession of 0.2‰ for δ15N-NO3ˉ and 0.4‰ for δ18O-NO3.ˉ The reproducibility of δ15NNO3ˉ and δ18O-NO3ˉ, based on long-run quality assurance tests, was ca. 0.22‰ and 0.51‰, respectively. The calibration of δ15N-NO3ˉ and δ18ONO3ˉ was done according to Casciotti and McIlvin (2007).
2.3 Data analysisApart from the Changjiang River runoff, the Changjiang estuary receives seawater from the Taiwan Warm Current that intrudes into the estuary along 50-m isobath and the Kuroshio Current from East China Sea surface (Zhu et al., 2004). Kuroshio waters are oligotrophic (Zhang et al., 2007) while the TWC waters contain regenerated nutrients. Consequently, a three-endmember mixing model was developed to estimate water sources in the CDW (identified by salinity and water temperature, Supplementary Fig.S1). Based on the identified water sources, calculation of the conservative distribution of NO3ˉ concentrations, isotopic compositions, as well as NH4+ concentrations were conducted (more details in Yan et al., 2017). According to the difference in concentration or isotope compositions between the value derived from the model estimation and the observation, offsets for solute concentrations or isotope compositions were obtained. The positive offset for solute concentration indicates the production behavior, while a negative value is a mirror of removal. For isotope compositions, the positive reflects heavy isotope enrichment and vice versa. In the present study, the water parameters derived from the XLJ station (Fig. 1), i.e. the upstream of both transects, were used to represent the Changjiang River water. For TWC water, the NO3ˉ concentration and related isotope compositions, as well as NH4+ concentration were from the bottom of the southeast sampling site in the same cruise (Fig. 1). For Kuroshio waters in the East China Sea, the NO3ˉ concentration was obtained from Wang et al. (2016) and NH4+ concentration from Zhang et al. (2007). These values are outlined in Supplementary Table S1. Statistical analyses, such as regression and student's t-test, were carried out for the measurement of variables using Minitab 17.0 software (Minitab Inc., Pennsylvania State University). The significant level for all analyses was assumed to be α=0.05. The spatial distribution of parameters along each transect was plotted in Surfer 14.0 (Golden Software Inc., USA) and the dot plots were done in Sigmaplot 12.5 (Systat Software Inc., USA).
3 RESULT 3.1 Water chemistry parametersSalinities of the cruise samples ranged from 0.11 to 34.5 on transect A (Fig. 2a). On transect B, a similar range (0.13-34.4) was observed (Fig. 2b). The salinity increased from the surface to bottom water. Additionally, the salinity difference between the surface and bottom increased from the river mouth to the outer plume, reaching ca. 8 due to strong stratification. The temperature of the Changjiang River surface water was approximately 26 ℃ and decreased to 18.9 ℃ at bottom water in the CDW outer plume (Fig. 2c). TSM concentration in the estuary water ranged from 9.0 to 75.6 mg/L. On transect A, TSM rapidly increased in the salinities of 5 to 10 regardless of seawater dilution. Afterwards, the TSM concentration sharply decreased, dropping to approximately 10 mg/L at the outermost site. Fluorescence intensities, an indicator of phytoplankton biomass, ranged from 0.34 to 12.8 μg/L in the estuary water. On both transects, fluorescence intensities were low when water salinities < 20. In the outer plume, fluorescence intensities rapidly increased and the watercolor darkened due to enrichment of phytoplankton, while the bottom water was fluorescence deficient (Fig. 2g-h). DO concentrations were ca. 6 mg/L at the inner sites (all depth). In the surface water, the DO concentration gradually increased and peaked at the sites with high fluorescence intensity. When water depth increased, the DO concentration in the saline CDW dropped (minimum 3.9 mg/L) (Fig. 2i-j).
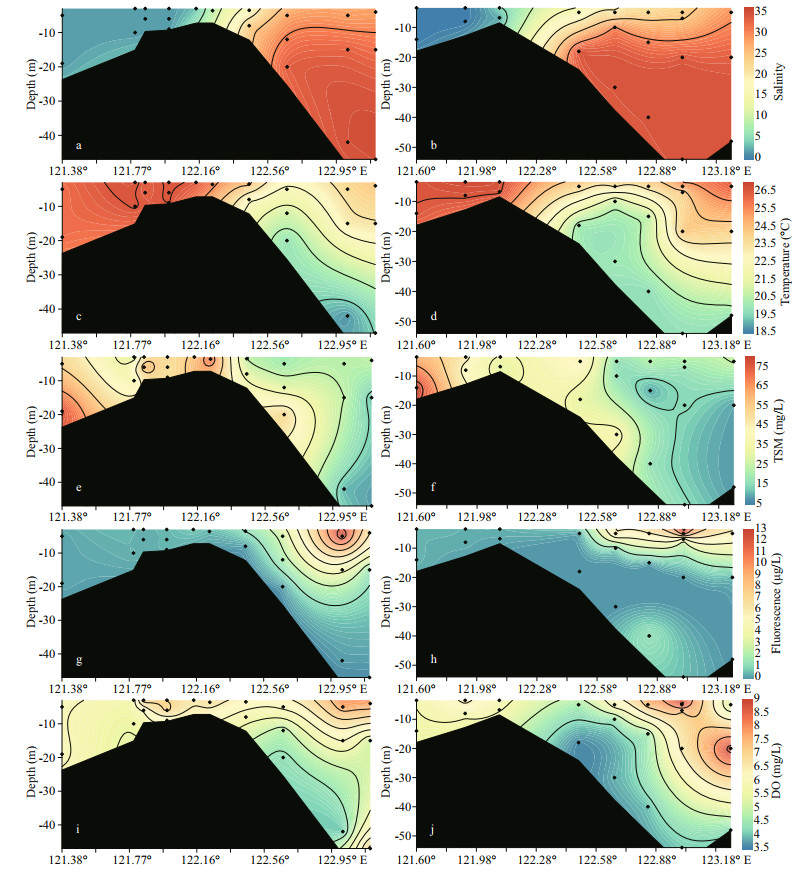
|
| Fig.2 Profile of salinity (a & b), temperature (c & d), TSM (e & f), fluorescence (g & h), and DO (i & j) on both transects The left panel shows the distribution on transect A while the right panel outlines the distribution on transect B. The profile extends from the river channel (ca. 121°E) to the CDW outer plume (ca. 123°E). The location of each site can be found in Fig. 1. |
NO3ˉ was the major DIN species, and the concentrations of NO3ˉ at the starting site (in the river channel) on transect A was approximately 119 μmol/L (Fig. 3a). Together with salinity increases, NO3ˉ decreased to ca. 5 μmol/L in the seawater surface (Fig. 4a). On transect B, NO3ˉ concentrations were ca. 108 μmol/L in river channels and dropped to 0.1 μmol/L in the outer plume (Fig. 3b). At the starting site, NO3ˉ concentrations in the surface and bottom waters were similar. The vertical difference in the NO3ˉ concentration increased in the outer plume with enhanced stratification. In contrast to NO3ˉ, NH4+ concentration in Changjiang River surface water was much lower (0.1-0.6 μmol/L). Concentrations of NH4+ increased to a maximum of 6.3 μmol/L at the higher salinity water (Fig. 3c-d).
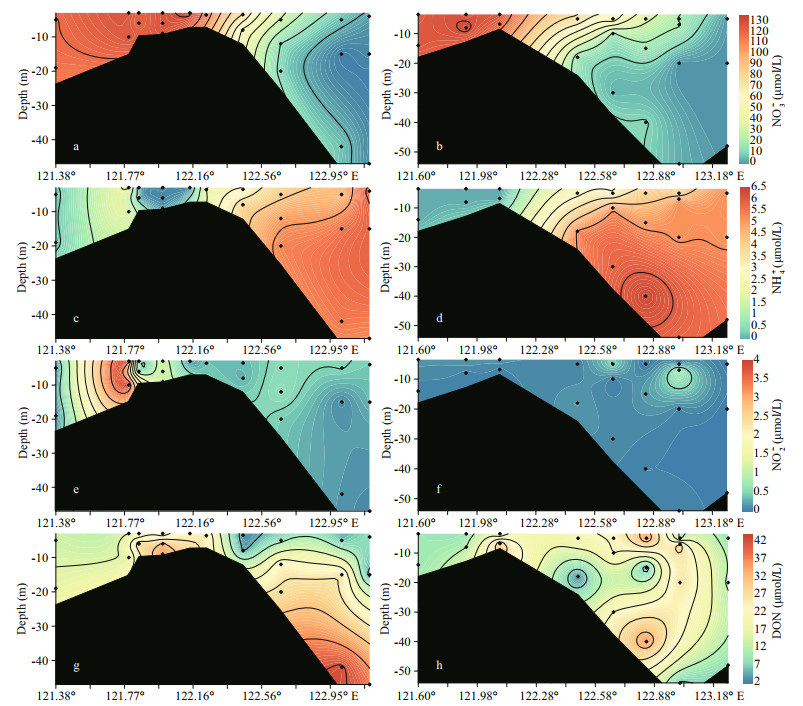
|
| Fig.3 Profile of NO3- (a & b), NH4+ (c & d), NO2ˉ, (e & f), and DON (g & h) on transect A (left panel) and transect B (right panel) The profile extends from the river channel (ca. 121°E) to the CDW outer plume (ca. 123°E). The location of each site can be found in Fig. 1. |
For the distribution of NO2ˉ, its concentration on both transects was frequently below 1 μmol/L, indicating its instability (Fig. 3e-f). The NO2ˉ concentration showed a peak near the river mouth on transect A (Fig. 3e), while no significant enrichment was found on transect B (Fig. 3f). The DON level varied between 5.5 and 42.6 μmol/L. In the Changjiang River runoff (salinity < 2), DON concentration was < 20 μmol/L (Fig. 3g-h). In the surface water on transect A (3-5-m depth), an increase in DON concentration, followed with decreases, was found. A similar peak in DON concentration was observed at the bottom of saline water (Fig. 3g). On transect B, three DON concentration peaks were identified, mainly occurred in the bottom water (10-40-m depth) (Fig. 3h).
Along transect A, δ15N-NO3ˉ ranged from 6.5‰ to 10.0‰ (Fig. 5a). In the surface water, the elevation of δ15N-NO3ˉ along the salinity gradient from the river mouth to the offshore areas was observed (Fig. 4a). In the outer plume of the CDW, δ15N-NO3ˉ decreased in the deeper water. The range of δ18O-NO3ˉ fell into -0.4‰ to 6.1‰ on transect A and displayed a similar trend along the salinity gradient as δ15N-NO3ˉ (Fig. 5c). On transect B, δ15N-NO3ˉ in the Changjiang River runoff was 6.6‰. In the high salinity CDW, the surface water manifested a significant enrichment in heavy isotopes, reaching 17.4‰ for δ15N-NO3ˉ (Fig. 5b). Nearly identical enrichment was found for δ18O-NO3ˉ, peaking at 14.7‰ (Fig. 5d). Unlike the surface water, both isotope compositions decreased in the bottom layers from the estuary toward the ocean. On both transects, positive correlations between δ15N/ δ18O-NO3ˉ and fluorescence intensity were observed (Fig. 4c-d). For the correlation between isotope compositions and ln(NO3ˉ) (natural logarithm of NO3ˉ concentration), the surface and bottom water clearly separated into two clusters (Fig. 4e-f), suggesting a stronger isotope fractionation in the surface water and regeneration in the bottom water. In addition, the slope of linear regression based on the surface water samples was large on transect B (Fig. 4f).
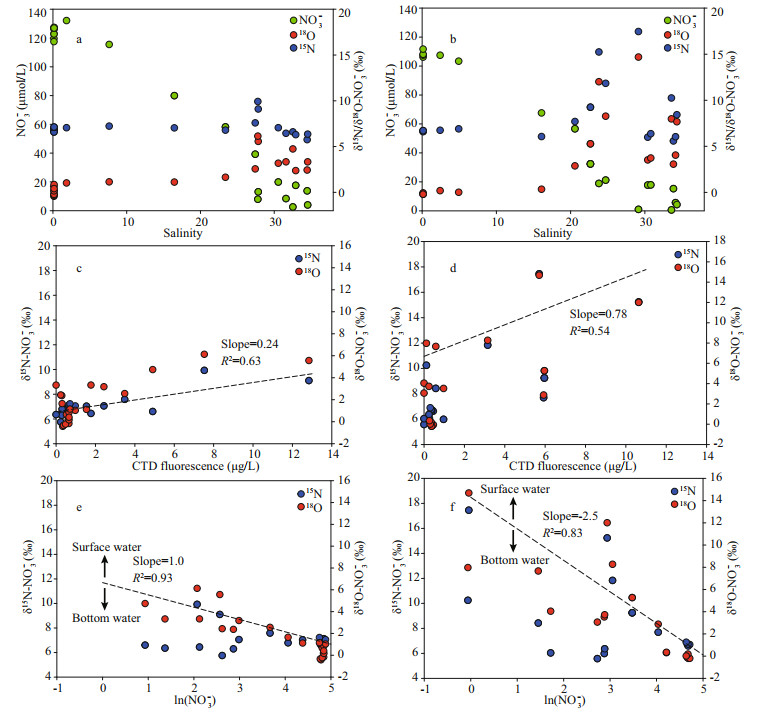
|
| Fig.4 Correlations between NO3- concentration and isotope compositions with salinity (a & b); correlation between stable isotope compositions in NO3- and fluorescence (c & d), and NO3- concentration (logarithm, e & f) Dash lines in c and d are regression between δ15N-NO3- and fluorescence. Dash lines in e and f are regression of δ15N-NO3- in the surface water. Left column was plots for transect A and right column was plots for transect B. |
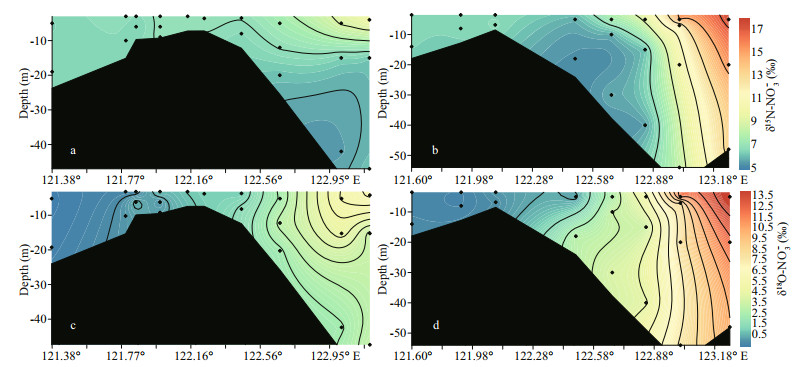
|
| Fig.5 Profile of δ15N-NO3- and δ18O-NO3- on transect A (a & c) and transect B (b & d) 3 |
The offset for NO3ˉ concentration, i.e. the difference in NO3ˉ concentration between the observed value and the values calculated from the three-endmember mixing model (Fig. 6a), was outlined along the salinity gradient (Fig. 6b, Supplementary Tables S2 & S3). On transect A, an increase in NO3ˉ concentration offset was found at inner stations (high turbidity water). In the outer plume, the offset for NO3ˉ concentration in the surface water was negative, suggesting an active consumption. The bottom water (high salinity) showed a patchy distribution and both increase and decrease in the offset values were obtained. Along transect B, offsets of NO3ˉ concentration in low salinity water (ca. 0-20) were frequently smaller than values obtained from transect B. For NH4+ (Fig. 6c), positive offsets were found in the outer plume, while negative values were observed in the low salinity water, especially on transect A. For isotope compositions, the water with the salinity below 20 showed a slightly positive offset for both δ15N/δ18O-NO3ˉ (Fig. 6d-e). In high salinity water, a positive offset in the surface was found while it decreased dramatically in the bottom water. The linear correlation between δ15N-NO3ˉ and δ18O-NO3ˉ was significant (slope=1.22, R2=0.90, P < 0.05)(Fig. 6f).
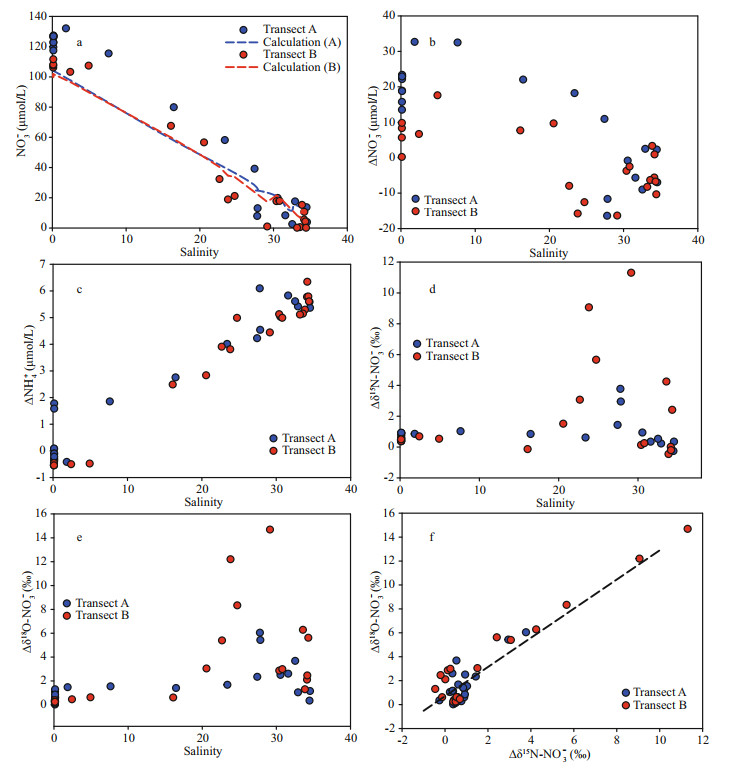
|
| Fig.6 Difference between model calculated NO3- concentration (dash line) and observation value (points) (a); distribution of NO3- (b), NH+4 (c), δ15N-NO3- (d), and δ18O-NO3- (e) offsets (Δ in the figure) along the salinity gradient; correlation between δ15N-NO3- and δ18O-NO3- offsets (f) |
In the past forty years, NO3ˉ concentrations in the Changjiang River runoff have markedly increased. At Datong station (600-km distance upstream of the first site on transect A), the NO3ˉ concentration increased from ca. 30 to 120 μmol/L (cf. Fig. 7a; data from Dai et al., 2011), accounting for the major component in the riverine DIN inventory (Fig. 3a-b). In the current study, the NO3ˉ concentration at the start of both transects (A: 119 μmol/L; B: 108 μmol/L) are comparable with published records from Yan et al. (2017). Enhanced NO3ˉ inventory in the Changjiang River has been attributed to weak biological assimilation due to high turbidity (Zhang et al., 2007) and great contributions from multiple sources, including chemical fertilizer leakage from terrestrial aquifers, wastewater discharge, atmospheric deposition and degradation of terrestrial organic nitrogen (Li et al., 2010; Dai et al., 2011).
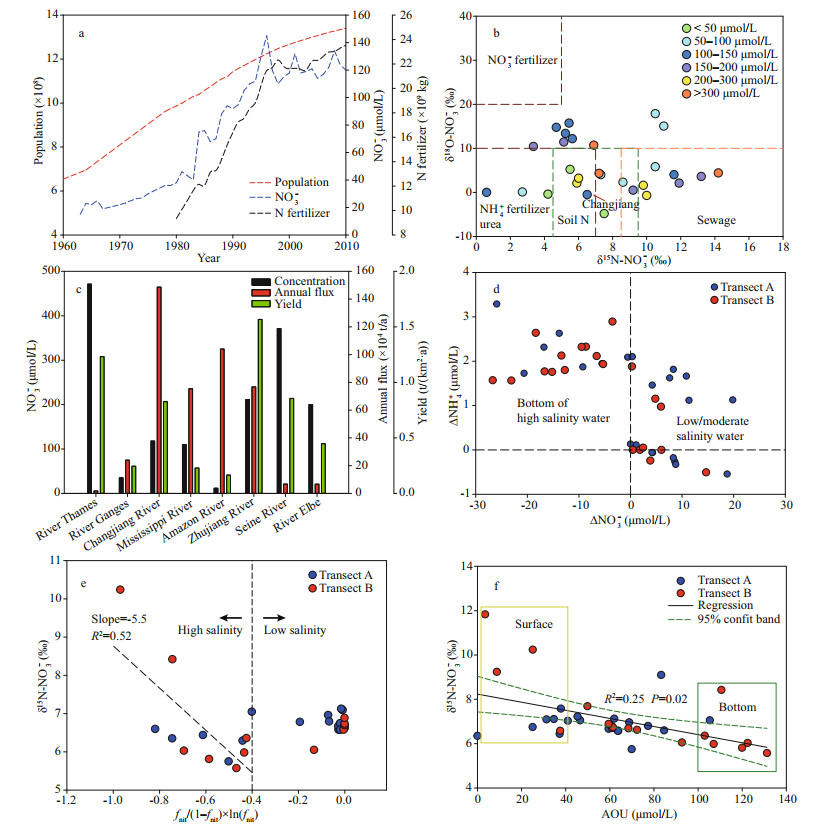
|
| Fig.7 Growth of Chinese population and N fertilizer application (China National Bureau of Statistics, http://www.stats.gov.cn/) and NO3- concentration in Changjiang River water mainly determinedat Datong Station (data mainly from Dai et al. (2011)) (a); distribution of δ15N/δ18O-NO3- in river water before discharge to estuaries (salinity < 0.5) on a global scale (b); comparison of NO3- concentration, annul flux, and watershed yield in different rivers on the global scale (c); correlation between NO3- concentration offset (Δ in the figure) and NH+4 concentration offset (Δ in the figure) in both transects (d); correlation of δ15N-NO3- in low salinity water (ca. salinity < 20) and bottom of high salinity water (20-34) with fnit/(1–fnit)×ln(fnit) (e); correlation between δ15N-NO3- and AOU (apparent oxygen utilization) in the CDW (f) In b, the isotope signals also highlight the potential sources for the riverine NO3-. The references of the displayed rivers can be found in Supplementary Table S4. The color used in the dot plots indicates the NO3- concentration in river water; in c, concentration data were obtained from Lohrenz et al. (1999), Sebilo et al. (2006), Santos et al. (2008), Bowes et al. (2012), Manna et al. (2013), and Cai et al. (2015). Annual discharge rate and area of the watershed were obtained from Milliman and Farnsworth (2011); in e, the dash line is the regression for the high salinity water. |
Given the isotope compositions in the Changjiang River runoff (outlined in Fig. 7b), both degradation of land-borne organic nitrogen (δ15N-NO3ˉ: 4.5‰ to 7.5‰; δ18O-NO3ˉ < 10‰) and leakage of chemical fertilizer (δ15N-NO3ˉ: 0 to 7‰; δ18O-NO3ˉ < 10‰) are likely to be important contributors (Chang et al., 2002; Li et al., 2010). In the Rajang River (Malaysia), the NO3ˉ concentration was 7.6 μmol/L and the major source of riverine NO3ˉ was the biological degradation of terrestrial organic matter due to the small population in its watershed (Jiang et al., 2019). In the St. Lawrence River (USA), another river with less anthropogenic disturbance, NO3ˉ concentration was 30.4 μmol/L, lower than that of Changjiang River (Thibodeau et al., 2013). Consequently, the proportion of NO3ˉ from organic matter decomposition may only account for a small portion in the riverine NO3ˉ inventory. Instead, fertilizer leakage, especially NH4+/ urea fertilizer, from terrestrial aquifers to the Changjiang River can be significant due to the intensive usage in agriculture to feed the large population. NO3ˉ enrichments were also found in the Seine River (France), Zhujiang (Pearl) River (China) and River Thames (UK), where substantial chemical fertilizer was applied to support the large population in the watershed (Sebilo et al., 2006; Bowes et al., 2012; Cai et al., 2015). The temporal correlation between N fertilizer application amount in China and NO3ˉ concentration in the Changjiang River water from the 1980s supported this conclusion (Fig. 7a).
Interestingly, in the upstream of Changjiang River (Zhangjiagang), both δ15N-NO3ˉ and δ18O-NO3ˉ were 8.3‰ and 2.6‰, respectively (Li et al., 2010), which were comparable to sewage-borne NO3ˉ enriched rivers, such as the Beijiang (China; Chen et al., 2009) and the Potomac River (USA; Pennino et al., 2016). In the river mouth, isotope compositions dropped. Such a significant decrease is likely due to active nitrification in the river water (Hsiao et al., 2014), which increases NO3ˉ concentration while decreases δ15N/δ18O-NO3ˉ (Sanders et al., 2018). The branches of the Changjiang River at the downstream, such as the Huangpu River, cover several megacities (e.g. Shanghai, Suzhou, Hangzhou). Different with the Changjiang River water, the mean concentration of NH4+ was similar to that of NO3ˉ in these branches (e.g. 1.4 mg/L for NH4+; 1.1 mg/L for NO3ˉ in the Huangpu River, Yang et al., 2007), suggesting the sewage loading. Coupled with nitrification, the sewage related NO3ˉ input from adjacent cities also requires attention from managers and stakeholders. In Fig. 7c, a global comparison among several rivers subject to intensive human activities was made. Due to the relatively large discharge rate (9.24×1011 m3/a; Milliman and Farnsworth, 2011), NO3ˉdelivered from the Changjiang River is on the top of the list (Fig. 7c), even the yield of NO3ˉ in the entire watershed was much smaller than the Zhujiang River and the River Thames. Currently, the Chinese government puts substantial efforts into restoring the land cover in the watershed and reducing the application of fertilizer (Huang et al., 2008); therefore, a gradual reduction in the river-borne NO3ˉ flux can be expected.
4.2 NO3ˉ production in the CDWIn the estuary, apart from potential errors introduced from sampling and irregular water mixing (e.g. small eddies), NO3ˉ addition was found in salinity < 20 (existing from the Changjiang River channels to the high turbidity water) and the outer plume (salinity >26), because of the positive offsets referenced to the conservative mixing status (Fig. 6b). The increment in NO3ˉ concentration at low/moderate salinity is in line with the findings reported by Liu et al. (2009) and Yao et al. (2014). The input from Changjiang River branches, especially the Huangpu River, to the Changjiang estuary might be the first reason for the increase. However, the NO3ˉ concentration in the Huangpu River was lower than the level in the Changjiang River runoff (Yang et al., 2007). More importantly, compared with the Changjiang River discharge rate in summer ((6-7)×104 m3/s), the Huangpu River loading was two orders of magnitude smaller (Zhu et al., 2018). Adding these together, the NO3ˉ concentration increase may not be the product of inputs from Changjiang River branches. In addition, the weak adsorption potential of NO3ˉ on particles in estuary water (Eyre, 1994) and limited variation in the adsorption-desorption balance from pH 5 to 9 (Zhang, 2007) indicate a minor influence from the adsorption-desorption process. Alternatively, the increase in NO3ˉ concentration may result from intensive nitrification, as highlighted in Fig. 8. On the one hand, the estuary directly receives riverine particles (Zhang et al., 2007) and microbes attached on particle surfaces. Given the high nitrification capability in the Changjiang River water, as aforementioned, it is not surprising to expect active nitrification on particle surface (Hsiao et al., 2014). On the other hand, seawater intrusion and desorption from the suspended particles, especially when salinity increases (Zhang, 2007), also add NH4+ for nitrification. Furthermore, TSM concentration at the freshwater endmember reached 55 mg/L. The proportion of N in TSM, measured by Gao et al. (2012) at XLJ, ranged from 0.12% to 0.24%, indicating a potential N source for NO3.ˉ Moreover, an increase in DON content in the high turbidity water was observed, likely due to degradation from particles or desorption, which benefits NO3ˉ production. For electron acceptors, DO was nearly saturated due to intensive mixing, which supported the transformation of organic N to NH4+ and eventually to NO3.ˉ Notably, the variation in δ15N in low salinity water was minor. In the Changjiang estuary, the reported mean δ15N in suspended particle matter measured at XLJ was 5.1‰ (Zhang et al., 2007) and 6.2 ‰ (Gao et al., 2012), which were in line with the δ15N-NO3ˉ value at the Changjiang River runoff (Fig. 4a-b). This similarity supported the reaction chain that includes organic nitrogen mineralization and nitrification, especially on particles (Fig. 8).
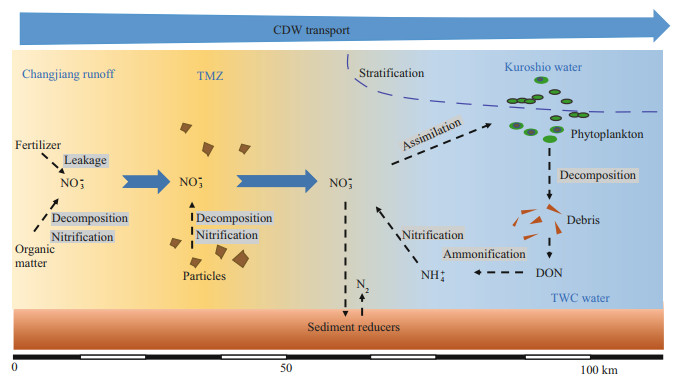
|
| Fig.8 Sketch of NO3- addition and removal in the CDW with a description of reaction pathways and water masses from the current cruise |
Intriguingly, between two transects, the concentration of NO2ˉ, the intermediate product in the nitrification reaction chain, differed in the low/ moderate salinity water. On transect A, the NO2ˉ concentration peak was observed near the river mouth (Fig. 3e), while an enrichment in NO2ˉ was not observed on transect B (Fig. 3f). Moreover, the peak value in positive NO3ˉ concentration offset obtained on transect A (ca. 32 μmol/L) was higher than the value on transect B (17 μmol/L). Apart from the bias introduced from the endmember selection, reasons for these differences may be linked to the TSM concentration. In particular, on transect A, TSM concentration markedly increased from the river water to brackish water (ca. salinity < 20) (Fig. 2e), and a large area of turbidity maximum zone (TMZ) was observed by a turbidity probe equipped on a towed system during the cruise, mainly due to relatively low discharge rate and strong tidal swash. The location of TMZ on transect A fits the historical observation (Yang et al., 2015). The marked increase in TSM concentration likely stimulates nitrification by providing substrates for remineralization (a positive link between TSM and NO3ˉ offsets in Supplementary Fig.S2). In addition, terrestrial particles in the TMZ were continuously suspended, prolonging the reaction time with oxidants, such as DO, in the water column. In contrast, a strong river plume rapidly injects and spreads in the East China Sea under the Coriolis force on transect B (Wu et al., 2010). Therefore, the typical TMZ along the transect was not captured during the cruise. Consequently, moderate NO3ˉ generation was observed because the particle-dependent nitrification may have been influenced by low concentrations of TSM.
Elevations in NO3ˉconcentration were also observed at the bottom of high salinity water (e.g. A9 and B6 in Supplementary Tables S2 & S3). Due to wide coverage of permeable sediments, it is possible to assume that submarine groundwater discharge may have contributed to this increase. Given the high concentration of NO3ˉ in groundwater, a small fraction could deeply change the NO3ˉ concentration in the receiving water (Moore, 2010). Generally, contaminated groundwater was enriched in δ18O-NO3ˉ due to continuous denitrification (15‰ to 90‰), as summarized by Xue et al. (2009). Those values are significantly higher than the records obtained in the present study, indicating that the high concentration of benthic NO3ˉ was not the result of groundwater injection. Alternatively, the increase in NO3ˉ concentration was triggered by bottom water nitrification (Fig. 8), which was supplied by the algae debris from the upper layer (Yan et al., 2017; Li et al., 2018). Compared to the nitrification rate in low salinity water, accumulation of NH4+ in the outer plume was found (Fig. 7d). Nitrification is conducted by ammonia-oxidation archaea (AOA) and ammoniaoxidation bacteria (AOB) and the community structure in ocean water differs from that of terrestrial water (Martens-Habbena et al., 2009). Usually, the marine-derived AOA (the dominant contributor to nitrification) show a higher affinity to NH4+ (Kuypers, 2017), indicating active nitrification at the high salinity CDW (Hsiao et al., 2014). Given the positive linkage between nitrification rate and NH4+ concentration in the CDW (Wang et al., 2018), minor accumulation of NH4+ should be observed, which is against the current observation. Moreover, the δ15NNO3ˉ fractionation was higher in the bottom of saline water than that in the low/moderate salinity CDW. In particular, the nitrification potential was introduced, which was defined as the slope between δ15N-NO3ˉ and fnit/(1-fnit)×ln(fnit), where fnit is the concentration ratio between NH4+ and DIN (Wang et al., 2017). This potential in the water column is positively linked to nitrification produced NH4+ in the benthic waters under the same substrate condition. It also helps the identification of reactant sources for nitrification process. Clearly, the bottom water at outer plume showed a significantly positive correlation between δ15N-NO3ˉ and fnit/(1-fnit)×ln(fnit) (Fig. 7e); whereas the trend in low salinity water was weak. The reason for such distribution can be attributed to the supply of oxidants. Near the river mouth, the shallow depth and extensive mixing lead to an active exchange of DO between the atmosphere and the estuary water. In the outer plume, stratification and enhanced water depth hampered DO exchanges, as evidenced by high values of apparent oxygen utilization (AOU) in the bottom water (Fig. 7f). In the CDW, a linear correlation between δ15N-NO3ˉ and AOU was observed (Fig. 7f), indicating the significance of DO in benthic nitrification. Furthermore, the suspended particles in the TMZ might contain high levels of trace metal, such as iron and magnesium, due to the adsorption and flocculation (Zhu et al., 2018). These metals could also act as the electron acceptor during nitrification (Hsiao et al., 2014); while the concentration is usually low in pelagic debris and saline water (Zhu et al., 2018). In summary, the DO supply and reactive trace metals constrained the nitrification rate in the bottom of saline water though reaction potentials can be high.
4.3 NO3ˉ removal in the CDWThe NO3ˉ removal appeared in two regions in the outer plume (Fig. 8). In the surface water, turbidity decreased at seaward of the TMZ, which resulted from stratification, reduced turbulence and vertical mixing, benefiting the light availability for phytoplankton. It agrees with the observed phytoplankton bloom in the CDW front (Gao and Song, 2005). Hence, the NO3ˉ consumption coupled with utilization by primary producers was observed, as depicted by the significantly positive correlation (R2>0.54) between fluorescence intensity and δ15NNO3ˉ (Fig. 4c-d). In the present study, marked increases in NO3ˉ isotope compositions occurred at the 20 isohaline (Fig. 4a-b), which was identical to the value from Zhang et al. (2015) on δ30Si enrichment in the estuary. Consequently, 20 isohaline can be treated as a starting boundary for the rapid uptake of nutrients by primary producers. As previously mentioned (Section 2.1), Skeletonema costatum was dominant in the outer plume on both transects. This assimilation increased isotope compositions, leading to strong correlations between NO3ˉ concentration and δ15N/ δ18O-NO3ˉ in surface water (Fig. 4e-f).
Though with identical phytoplankton species, NO3ˉ depletion (below 1 μmol/L) was observed on transect B (Fig. 4b). In comparison, approximately 5 μmol/L NO3ˉ still existed at the surface of transect A. Moreover, the correlation slopes of δ15N/δ18O-NO3ˉ to ln(NO3ˉ) in the surface water were higher on transect B (Fig. 4f), indicating strong isotopic fractionation during the consumption of NO3.ˉ The surface water is usually limited in denitrification carriers due to high DO content (Wong et al., 2014). Moreover, the fluorescence intensity and water residence time on both transects were similar. Adding these together, the difference in the stable isotope composition between transects A and B in this study likely results from strong stratification outside of the TMZ. The potential energy anomaly, an indicator of stratification, displays a peak on transect B (Supplementary Fig.S3), indicating a slow water exchange between the surface and bottom. Such strong stability is beneficial for the growth of phytoplankton in the surface water, which may have resulted in the continuous utilization of NO3ˉ and elevation of δ15N/δ18O-NO3ˉ in the CDW plume. Interestingly, the enhanced NO3ˉ utilization by Skeletonema costatum did not trigger a significant increase in biomass, as outlined by the similar CTD fluorescence intensity between transects A and B (Fig. 2g-h). One reason is that strong currents in the Changjiang River plume had flushed the phytoplankton offshore. More importantly, this phenomenon may be linked to density effect, which suggests that algae concentration could not continuously increase; whereas algae communities, especially for the nutrient-sensitive species, such as Skeletonema costatum (Li et al., 2018), could continuously accumulate nutrient from ambient environment into cells (luxury consumption). Collos et al. (1992) revealed that intracellular accumulation of NO3ˉ in Skeletonema costatum reached 17 mmol/L, i.e. several orders of magnitude higher than the ambient NO3ˉ concentration. The excess N may be stored in the vacuole of the algae as amino acids or protein (Ketchum, 1939). When N concentration in the ambient environment decreases, the stored N could sustain the cell function and algae biomass, leading to a continuous bloom in the CDW. In the benthic water, decrease in NO3ˉ concentration was also observed (Fig. 3a-b). The concentration decrease mainly results from denitrification since Skeletonema costatum cannot actively move to the bottom water due to lack of flagellates (Li et al., 2018). Denitrification could occur in both the water column and sediments (Yan et al., 2017). Usually, 2 mg/L DO concentration was assumed to be the highest level that denitrifiers could bear in seawater (Codispoti and Christensen, 1985). The DO concentration in the present study was higher than this threshold. Therefore, sediments are the major rector for denitrification. In the Changjiang estuary, high denitrification potential in benthic and intertidal sediments, especially in cohesive sediments (muddy sediment), has been reported (Song et al., 2013). Coupled with exchanges between sediment porewater and overlying water, a significant fraction of NO3ˉ was injected into sediments, especially the permeable sediments. Compared with the biological assimilation, denitrification in the sediment may not significantly alter stable isotope values (< 0.5‰ here) because of the completed transformation (Yan et al., 2017). Moreover, sediment denitrification might be coupled with nitrification (Fig. 8), effectively removing the produced NO3ˉ and hence preventing the transport of NO3ˉ from the deep water to surface water.
5 CONCLUSIONIn the present study, the source of NO3ˉ in the Changjiang River runoff before the river-sea mixing was mainly attributed to inputs from chemical fertilizer (based on δ15N/δ18O-NO3ˉ values), indicating the importance of management on fertilizer application and reforestation in the watershed. Sewage-related NO3ˉ may also be important to the riverine inventory while nitrification alters the isotope compositions. In the coastal zone that receives the Changjiang River runoff, when salinities < 20, positive offsets for NO3ˉ concentration were obtained, likely due to the strong nitrification supplied by remineralization of N on particles and DON. The NO3ˉ production peaked at the TMZ because of resuspension of particles and high levels of DO. In the high salinity CDW (salinity >20), the surface water became a sink for NO3ˉ due to diatom assimilation. Consequently, 20 isohaline can be regarded as a boundary for the rapid uptake of nutrients by primary producers, benefiting the predication of algae bloom in coastal management. Compared with transect A, the strong stratification at the outer plume of transect B markedly stimulated phytoplankton utilization. The boost in the biological assimilation did not increase diatom biomass, therefore the assimilation may be linked to phytoplankton luxury consumption. In the bottom water, denitrification and nitrification coexisted. Nitrification was supplied by the degradation of algae debris, which increased NO3ˉ concentrations but decreased stable isotope compositions. Denitrification likely occurred at the water-sediment interface that continuously removed NO3ˉ, but without significant alternation on NO3ˉ due to completed transformation. The production and removal for NO3ˉ in the CDW should be taken into consideration by coastal managers and modeling workers. Furthermore, microorganism analyses and incubation experiments to quantitatively determine the dynamic between environmental drivers and NO3ˉaddition/removal rates are necessary.
6 DATA AVAILABILITY STATEMENTThe data used in the present study are available from the corresponding author upon request.
7 ACKNOWLEDGMENTTechnical support by Prof. GAO Yonghui, Prof. LI Bo, Ms. ZHENG Wei, Dr. ZHU Xunchi, Ms. QI Lijun, Mr. LIU Zhengbo, Ms. CAO Wanwan, Ms. JIANG Shuo, Mr. ZHU Kun, Mr. DAI Jinlong, and the R/V Zheyuke Ⅱ crew during the cruise are acknowledged. The authors also appreciate the great assistance from Prof. YU Zhiming at the Institute of Oceanology, Chinese Academy of Sciences for the assistance in stable isotope analyses. Thanks are also due to the editor and anonymous reviewers, whose comments helped to improve the original manuscript.
Supplementary material (Supplementary Tables S1–S4, Figs.S1–S3) is available in the online version of this article at https://doi.org/10.1007/s00343-020-0149-8.
Bowes M J, Ings N L, McCall S J, Warwick A, Barrett C, Wickham H D, Harman S A, Armstrong L K, Scarlett P M, Roberts C, Lehmann K, Sing A C. 2012. Nutrient and light limitation of periphyton in the River Thames: implications for catchment management. Science of the Total Environment, 434: 201-212.
DOI:10.1016/j.scitotenv.2011.09.082 |
Cai P H, Shi X M, Hong Q Q, Li Q, Liu L F, Guo X H, Dai M H. 2015. Using 224Ra/228Th disequilibrium to quantify benthic fluxes of dissolved inorganic carbon and nutrients into the Pearl River Estuary. Geochimica et Cosmochimica Acta, 170: 188-203.
DOI:10.1016/j.gca.2015.08.015 |
Casciotti K L, McIlvin M R. 2007. Isotopic analyses of nitrate and nitrite from reference mixtures and application to Eastern Tropical North Pacific waters. Marine Chemistry, 107(2): 184-201.
DOI:10.1016/j.marchem.2007.06.021 |
Chang C C Y, Kendall C, Silva S R, Battaglin W A, Campbell D H. 2002. Nitrate stable isotopes: tools for determining nitrate sources among different land uses in the Mississippi River Basin. Canadian Journal of Fisheries and Aquatic Sciences, 59(12): 1 874-1 885.
DOI:10.1139/f02-153 |
Chang P H, Isobe A, Kang K R, Ryoo S B, Kang H S, Kim Y H. 2014. Summer behavior of the Changjiang diluted water to the East/Japan Sea: a modeling study in 2003. Continental Shelf Research, 81: 7-18.
DOI:10.1016/j.csr.2014.03.007 |
Chen F J, Jia G D, Chen J Y. 2009. Nitrate sources and watershed denitrification inferred from nitrate dual isotopes in the Beijiang River, South China. Biogeochemistry, 94(2): 163-174.
DOI:10.1007/s10533-009-9316-x |
Codispoti L A, Christensen J P. 1985. Nitrification, denitrification and nitrous oxide cycling in the eastern tropical South Pacific Ocean. Marine Chemistry, 16(4): 277-300.
DOI:10.1016/0304-4203(85)90051-9 |
Collos Y, Siddiqi M Y, Wang M Y, Glass A D M, Harrison P J. 1992. Nitrate uptake kinetics by two marine diatoms using the radioactive tracer 13N. Journal of Experimental Marine Biology and Ecology, 163(2): 251-260.
DOI:10.1016/0022-0981(92)90053-D |
Dai Z J, Du J Z, Zhang X L, Su N, Li J F. 2011. Variation of riverine material loads and environmental consequences on the Changjiang (Yangtze) estuary in recent decades(1955-2008). Environmental Science & Technology, 45(1): 223-227.
DOI:10.1021/es103026a |
Domangue R J, Mortazavi B. 2018. Nitrate reduction pathways in the presence of excess nitrogen in a shallow eutrophic estuary. Environmental Pollution, 238: 599-606.
DOI:10.1016/j.envpol.2018.03.033 |
Ebina J, Tsutsui T, Shirai T. 1983. Simultaneous determination of total nitrogen and total phosphorus in water using peroxodisulfate oxidation. Water Research, 17(12): 1 721-1 726.
DOI:10.1016/0043-1354(83)90192-6 |
Edmond J M, Spivack A, Grant B C, Hu M H, Chen Z, Chen S, Zeng X. 1985. Chemical dynamics of the Changjiang Estuary. Continental Shelf Research, 4(1-2): 17-36.
DOI:10.1016/0278-4343(85)90019-6 |
Eyre B. 1994. Nutrient biogeochemistry in the tropical Moresby River Estuary system North Queensland, Australia. Estuarine, Coastal and Shelf Science, 39(1): 15-31.
DOI:10.1006/ecss.1994.1046 |
Galloway J N, Dentener F J, Capone D G, Boyer E W, Howarth R W, Seitzinger S P, Asner G P, Cleveland C C, Green P A, Holland E A, Karl D M, Michaels A F, Porter J H, Townsend A R, Vöosmarty C J. 2004. Nitrogen cycles: past, present, and future. Biogeochemistry, 70(2): 153-226.
DOI:10.1007/s10533-004-0370-0 |
Gao L, Li D J, Zhang Y W. 2012. Nutrients and particulate organic matter discharged by the Changjiang (Yangtze River): seasonal variations and temporal trends. Journal of Geophysical Research-Biogeosciences, 117(G4): G04001.
DOI:10.1029/2012JG001952 |
Gao X L, Song J M. 2005. Phytoplankton distributions and their relationship with the environment in the Changjiang Estuary, China. Marine Pollution Bulletin, 50(3): 327-335.
DOI:10.1016/j.marpolbul.2004.11.004 |
Granger J, Sigman D M, Rohde M M, Maldonado M T, Tortell P D. 2010. N and O isotope effects during nitrate assimilation by unicellular prokaryotic and eukaryotic plankton cultures. Geochimica et Cosmochimica Acta, 74(3): 1 030-1 040.
DOI:10.1016/j.gca.2009.10.044 |
Hansen H P, Koroleff F. 1999. Determination of nutrients. In: Grasshoff K, Kremling K, Ehrhardt M eds. Methods of Seawater Analysis. Wiley, Washington. p. 159–228.
|
Hsiao S S Y, Hsu T C, Liu J W, Xie X, Zhang Y, Lin J, Wang H, Yang J Y T, Hsu S C, Dai M, Kao S J. 2014. Nitrification and its oxygen consumption along the turbid Chang Jiang River plume. Biogeosciences, 11(7): 2-2 098.
DOI:10.5194/bg-11-2083-2014 |
Huang J K, Hu R F, Cao J M, Rozelle S. 2008. Training programs and in-the-field guidance to reduce China's overuse of fertilizer without hurting profitability. Journal of Soil and Water Conservation, 63(5): 165A-167A.
DOI:10.2489/jswc.63.5.165A |
Jiang S, Müller M, Jin J, Wu Y, Zhu K, Zhang G S, Mujahid A, Rixen T, Muhamad M F, Sia E S A, Jang F H A, Zhang J. 2019. Dissolved inorganic nitrogen in a tropical estuary in Malaysia: transport and transformation. Biogeosciences, 16(14): 2 821-2 836.
DOI:10.5194/bg-16-2821-2019 |
Justić D, Turner R E, Rabalais N N. 2003. Climatic influences on riverine nitrate flux: implications for coastal marine eutrophication and hypoxia. Estuaries, 26(1): 1-11.
DOI:10.1007/BF02691688 |
Ketchum B H. 1939. The absorption of phosphate and nitrate by illuminated cultures of Nitzschia closterium. American Journal of Botany, 26(6): 399-407.
DOI:10.1002/j.1537-2197.1939.tb09293.x |
Kuypers M M M, Marchant H K, Kartal B. 2018. The microbial nitrogen-cycling network. Nature Reviews Microbiology, 16(5): 263-276.
DOI:10.1038/nrmicro.2018.9 |
Kuypers M M M. 2017. Microbiology: a fight for scraps of ammonia. Nature, 549(7671): 162-163.
DOI:10.1038/549162a |
Li S L, Liu C Q, Li J, Liu X L, Chetelat B, Wang B L, Wang F S. 2010. Assessment of the sources of nitrate in the Changjiang River, China using a nitrogen and oxygen isotopic approach. Environmental Science & Technology, 44(5): 1 573-1 578.
DOI:10.1021/es902670n |
Li Z, Song S Q, Li C W, Yu Z M. 2018. The sinking of the phytoplankton community and its contribution to seasonal hypoxia in the Changjiang (Yangtze River) estuary and its adjacent waters. Estuarine, Coastal and Shelf Science, 208: 170-179.
DOI:10.1016/j.ecss.2018.05.007 |
Liu S M, Qi X H, Li X N, Ye H R, Wu Y, Ren J L, Zhang J, Xu W Y. 2016. Nutrient dynamics from the Changjiang(Yangtze River) estuary to the East China Sea. Journal of Marine Systems, 154: 15-27.
DOI:10.1016/j.jmarsys.2015.05.010 |
Liu X J, Yu Z M, Song X X, Cao X H. 2009. The nitrogen isotopic composition of dissolved nitrate in the Yangtze River (Changjiang) estuary, China. Estuarine, Coastal and Shelf Science, 85(4): 641-650.
DOI:10.1016/j.ecss.2009.09.017 |
Lohrenz S E, Fahnenstiel G L, Redalje D G, Lang G A, Dagg M J, Whitledge T E, Dortch Q. 1999. Nutrients, irradiance, and mixing as factors regulating primary production in coastal waters impacted by the Mississippi River plum. Continental Shelf Research, 19(9): 1 113-1 141,.
DOI:10.1016/S0278-4343(99)00012-6 |
Loken L C, Small G E, Finlay J C, Sterner R W, Stanley E H. 2016. Nitrogen cycling in a freshwater estuary. Biogeochemistry, 127(2-3): 199-216.
DOI:10.1007/s10533-015-0175-3 |
Manna R K, Satpathy B B, Roshith C M, Naskar M, Bhaumik U, Sharma A P. 2013. Spatio-temporal changes of hydrochemical parameters in the estuarine part of the River Ganges under altered hydrological regime and its impact on biotic communities. Aquatic Ecosystem Health & Management, 16(4): 433-444.
DOI:10.1080/14634988.2013.853596 |
Martens-Habbena W, Berube P M, Urakawa H, de la Torre J R, Stahl D A. 2009. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature, 461(7266): 976-979.
DOI:10.1038/nature08465 |
Milliman J D, Farnsworth K L. 2011. River Discharge to the Coastal Ocean-A Global Synthesis. Cambridge University Press, London. 319p.
|
Moore W S. 2010. The effect of submarine groundwater discharge on the ocean. Annual Review of Marine Science, 2: 59-88.
DOI:10.1146/annurev-marine-120308-081019 |
Pennino M J, Kaushal S S, Murthy S N, Blomquist J D, Cornwell J C, Harris L A. 2016. Sources and transformations of anthropogenic nitrogen along an urban river-estuarine continuum. Biogeosciences, 13(22): 6 211-6 228.
DOI:10.5194/bg-13-6211-2016 |
Sanders T, Schöl A, Dähnke K. 2018. Hot spots of nitrification in the Elbe Estuary and their impact on nitrate regeneration. Estuaries and Coasts, 41(1): 128-138.
DOI:10.1007/s12237-017-0264-8 |
Santos M L S, Muniz K, Barros-Neto B, Araujo M. 2008. Nutrient and phytoplankton biomass in the Amazon River shelf waters. Anais da Academia Brasileira de Ciências, 80(4): 703-717.
DOI:10.1590/S0001-37652008000400011 |
Sebilo M, Billen G, Mayer B, Billiou D, Grably M, Garnier J, Mariotti A. 2006. Assessing nitrification and denitrification in the Seine River and estuary using chemical and isotopic techniques. Ecosystems, 9(4): 564-577.
DOI:10.1007/s10021-006-0151-9 |
Sigman D M, Casciotti K L, Andreani M, Barford C, Galanter M, Böhlke J K. 2001. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Analytical Chemistry, 73(17): 4 145-4 153.
DOI:10.1021/ac010088e |
Song G D, Liu S M, Marchant H, Kuypers M M M, Lavik G. 2013. Anammox, denitrification and dissimilatory nitrate reduction to ammonium in the East China Sea sediment. Biogeosciences, 10(11): 6 851-6 864.
DOI:10.5194/bg-10-6851-2013 |
Thibodeau B, Hélie J F, Lehmann M F. 2013. Variations of the nitrate isotopic composition in the St. Lawrence River caused by seasonal changes in atmospheric nitrogen inputs. Biogeochemistry, 115: 287-298.
DOI:10.1007/s10533-013-9834-4 |
Wang W T, Yu Z M, Song X X, Wu Z X, Yuan Y Q, Zhou P, Cao X H. 2016. The effect of Kuroshio Current on nitrate dynamics in the southern East China Sea revealed by nitrate isotopic composition. Journal of Geophysical Research: Oceans, 121(9): 7 073-7 087.
DOI:10.1002/2016JC011882 |
Wang W T, Yu Z M, Song X X, Wu Z X, Yuan Y Q, Zhou P, Cao X H. 2017. Characteristics of the δ15NNO3distribution and its drivers in the Changjiang River estuary and adjacent waters. Chinese Journal of Oceanology and Limnology, 35(2): 367-382.
DOI:10.1007/s00343-016-5276-x |
Wang W T, Yu Z M, Wu Z X, Song S Q, Song X X, Yuan Y Q, Cao X H. 2018. Rates of nitrification and nitrate assimilation in the Changjiang River estuary and adjacent waters based on the nitrogen isotope dilution method. Continental Shelf Research, 163: 35-43.
DOI:10.1016/j.csr.2018.04.014 |
Weigand M A, Foriel J, Barnett B, Oleynik S, Sigman D M. 2016. Updates to instrumentation and protocols for isotopic analysis of nitrate by the denitrifier method. Rapid Communications in Mass Spectrometry, 30(12): 1 365-1 383.
DOI:10.1002/rcm.7570 |
Wong W W, Grace M R, Cartwright I, Cook P L M. 2014. Sources and fate of nitrate in a groundwater-fed estuary elucidated using stable isotope ratios of nitrogen and oxygen. Limnology and Oceanography, 59(5): 1 493-1 509.
DOI:10.4319/lo.2014.59.5.1493 |
Wu H, Zhu J R, Choi B H. 2010. Links between saltwater intrusion and subtidal circulation in the Changjiang Estuary: a model-guided study. Continental Shelf Research, 30(17): 1 891-1 905.
DOI:10.1016/j.csr.2010.09.001 |
Xue D M, Botte J, De Baets B, Accoe F, Nestler A, Taylor P, Van Cleemput O, Berglund M, Boeckx P. 2009. Present limitations and future prospects of stable isotope methods for nitrate source identification in surface- and groundwater. Water Research, 43(5): 1 159-1 170.
DOI:10.1016/j.watres.2008.12.048 |
Yan X L, Xu M N, Wan X S, Yang J Y T, Trull T W, Dai M H, Kao S J. 2017. Dual isotope measurements reveal zoning of nitrate processing in the summer Changjiang (Yangtze) river plume. Geophysical Research Letters, 44(24): 12 289-12 297.
DOI:10.1002/2017GL075951 |
Yang H J, Shen Z M, Zhang J P, Wang W H. 2007. Water quality characteristics along the course of the Huangpu River (China). Journal of Environmental Sciences, 19(10): 1 193-1 198.
DOI:10.1016/S1001-0742(07)60195-8 |
Yang Y P, Zhang M J, Li Y T, Zhang W. 2015. The variations of suspended sediment concentration in Yangtze River Estuary. Journal of Hydrodynamics, 27(6): 845-856.
DOI:10.1016/s1001-6058(15)60547-9 |
Yao Q Z, Yu Z G, Li L L, Chen HT, Mi T Z. 2014. Transformation and source of nutrients in the Changjiang Estuary. Science China Chemistry, 57(5): 779-790.
DOI:10.1007/s11426-013-5040-4 |
Yu H Y, Yu Z M, Song X X, Cao X H, Yuan Y Q, Lu G Y. 2015. Seasonal variations in the nitrogen isotopic composition of dissolved nitrate in the Changjiang River estuary, China. Estuarine, Coastal and Shelf Science, 155: 148-155.
DOI:10.1016/j.ecss.2015.01.017 |
Zhang A Y, Zhang J, Hu J, Zhang R F, Zhang G S. 2015. Silicon isotopic chemistry in the Changjiang Estuary and coastal regions: impacts of physical and biogeochemical processes on the transport of riverine dissolved silica. Journal of Geophysical Research-Oceans, 120(10): 6 943-6 957.
DOI:10.1002/2015JC011050 |
Zhang A. 2007. Study on the Controlling of Nutrient Phase Transformation by Adsorption-Desorption in the Chanjiang Estuary. East China Normal University, Shanghai, China. 168p.
|
Zhang J, Wu Y, Jennerjahn T C, Ittekkot V, He Q. 2007. Distribution of organic matter in the Changjiang (Yangtze River) Estuary and their stable carbon and nitrogen isotopic ratios: implications for source discrimination and sedimentary dynamics. Marine Chemistry, 106(1-2): 111-126.
DOI:10.1016/j.marchem.2007.02.003 |
Zhang J. 1996. Nutrient elements in large Chinese estuaries. Continental Shelf Research, 16(8): 1 023-1 045.
DOI:10.1016/0278-4343(95)00055-0 |
Zhu J R, Chen C S, Ding P X, Li C Y, Lin H C. 2004. Does the Taiwan warm current exist in winter?. Geophysical Research Letters, 31(12): L12302.
DOI:10.1029/2004gl019997 |
Zhu X C, Zhang R F, Wu Y, Zhu J R, Bao D Y, Zhang J. 2018. The remobilization and removal of Fe in Estuary-a case study in the Changjiang Estuary, China. Journal of Geophysical Research-Oceans, 123(4): 2 539-2 553.
DOI:10.1002/2017JC013671 |
 2021, Vol. 39
2021, Vol. 39


